1. INTRODUCTION
According to WHO, an estimated 1.13 billion people worldwide have high blood pressure in 2019 [1]. In Vietnam, according to the 2015 national survey report, one in five adults has hypertension, the number of hypertensive patients is estimated at 12 million people [2]. Early detection and good control of blood pressure reduce the risk of complications and mortality from cardiovascular diseases. Despite the wide variety of antihypertensive drugs available, the proportion of hypertensive patients who are treated and have blood pressure under control (usually <140/90 mmHg) remains low. In the treatment of hypertension, monotherapy is difficult to control blood pressure well because hypertension is caused by a combination of many complex mechanisms. Various clinical trials [3-5] have demonstrated that the majority of patients need to be treated with multiple antihypertensive agents with distinct mechanisms of action to achieve and maintain blood pressure goals, in which one-third of patients required three or more antihypertensive agents [6]. Although combination therapy two of three active ingredients telmisartan, amlodipine besylate, and hydrochlorothiazide has been shown to provide positive benefits in blood pressure control and patient tolerability, the combination therapy of all three agents have been shown to be effective in moderate to severe hypertension, with a significant additional reduction in blood pressure compared with dual use [7].
The fixed dose of a combination of two or three drugs in one antihypertensive drug product has been researched, manufactured, and widely used in the market by pharmaceutical companies. In which, the combination of amlodipine besylate, telmisartan and hydrochlorothiazide has proven superiority in the treatment of hypertension, contributing to helping patients adhere to the treatment regimen [8]. However, the combination of these three active ingredients is a challenge in drug testing due to the physico-chemical properties, UV spectrum, as well as the different content of active ingredients in the tablets. Therefore, the simultaneous quantification of these active ingredients encounters some difficulties in sample preparation, selection of detection wavelengths, finding analytical conditions, etc.
Up to now, there has not had any monograph in the Vietnamese Pharmacopoeia V and the reference pharmacopoeias for quality control for this combination drug. In particular, the US Pharmacopoeia (USP-NF 2021) has a monograph for the combination of telmisartan - amlodipine besylate, and telmisartan - hydrochlorothiazide. In Vietnam, there is still no published work on the simultaneous quantification of these three active ingredients.
Therefore, the development of a procedure for simultaneous quantification of these three active ingredients in the preparation makes an important contribution to the establishment of in-house specification, helps to quickly and accurately test drug quality, reduces the time required for drug quality control and effort when quantifying each component separately.
2. MATERIALS AND METHOD
Tablets supplied by R&D Department - Pharmaceutical Joint Stock Company X. Each tablet contains: Amlodipine (AML) 5 mg, Hydrochlorothiazide (HCT) 12.5 mg, Telmisartan (TEL) 40 mg, excipients just enough 1 tablet.
Reference standards: amlodipine besylate batch number R09060, content 99.9%, telmisartan batch number R085R0, content 99.6%, hydrochlorothiazide batch number J1F070, content 99.7%, origin of USP.
Solvents and chemicals: LC-grade methanol, acetonitrile (ACN); ammonium dihydrophosphate, concentrated phosphoric acid were of analytical grade.
Research equipment: Analytical equipment is a Waters Alliane e2695 high performance liquid chromatography system, a Waters 2996 PDA probe, a Metler Toledo S20 pH meter, a Sartorius CPD225D 5-digit analytical balance, a column Xterra C18 (250 mm × 4.6 mm, 5 μm) – USA. Precision glassware for analytical use.
Based on the chemical structure and UV absorption spectrum of the active ingredients, the published quantitative works [9-16], and the monographs of each active ingredient in the pharmacopoeias, high-performance liquid chromatography (HPLC) reverse phase with PDA probe and mobile phase with buffer solution were selected.
In this study, we have investigated the chromatographic conditions as shown in the following table:
| Chromato graphic conditions | Mobile phase |
|---|---|
| 1 | ACN - water phosphoric acid 0.01% at the ratio of 40:60 (v/v) |
| 2 | ACN - water phosphoric acid 0.01% at the ratio of 30:70 (v/v) |
| 3 | ACN - ammonium dihydrophosphate buffer pH 3.0 at the ratio of 40:60 (v/v) |
| 4 | ACN - ammonium dihydrophosphate buffer pH 3.0 at the ratio of 30:70 (v/v) |
| 5 | Methanol–ammonium dihydrophosphate buffer pH 3.0 at the ratio of 40:60 |
| 6 | The gradient elution program (1) shown in Table 2 |
| 7 | The gradient elution program (2) shown in Table 3 |
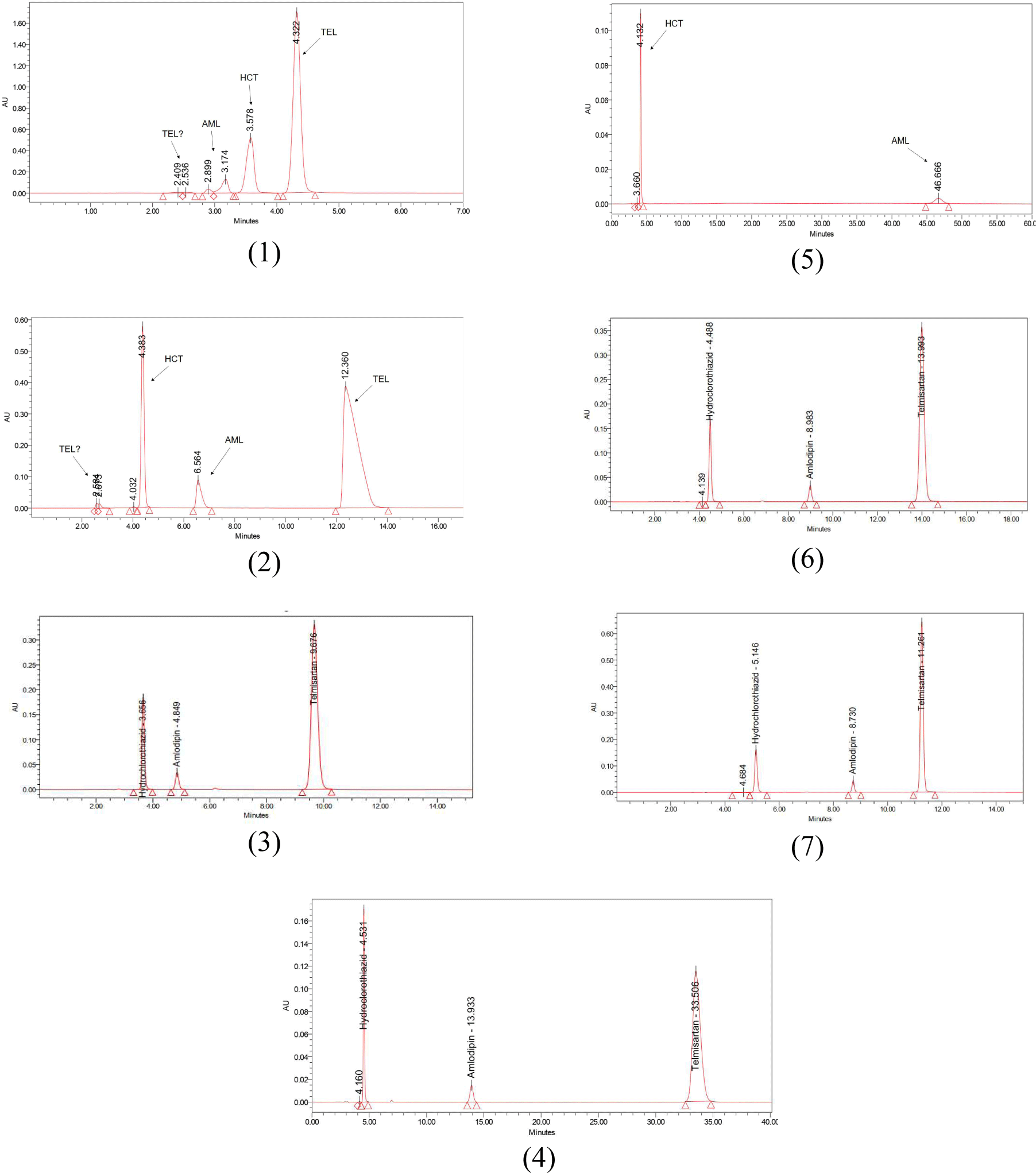
After investigating different chromatographic conditions, we selected suitable chromatographic conditions: The three peaks of amlodipine, telmisartan and hydrochlorothiazide are completely separated in the chromatogram (RS ≥ 1.5) with required purity, tailing factors ranged from 0.8 to 1.5, theoretical plate number ≥ 2000.
3. RESULTS
In chromatographic condition 1, the active substances are eluted early with the retention time of all peaks less than 5 min, in general the peaks have not separated completely from each other. Peak amlodipine is irregular in shape with two absorption peaks at 2.899 min (min) and 3.174 min and has not reached purity.
In chromatographic condition 2, the chromatogram showed a clear separation between the peaks. The retention time of the peaks has improved clearly, the peaks elute slower than in the condition 1. Specifically, the hydrochlorothiazide peak has a retention time increased from 3.578 min → 4.383 min, however, the hydrochlorothiazide peak has not reached the purity and there is an impurity peak of hydrochlorothiazide at 4.032 min. Next, the amlodipine peak has a retention time increasing from 3.174 min to 6.564 min and no longer splits, has a high tailing factor (1.7).
In chromatographic condition 3, the elution time of the active peaks was improved compared with the condition 1. For the hydrochlorothiazide peak, the retention time increased from 3.578 min → 3.656 min, the capacity factor was still low (K = 0.4), and from the UV spectrum results, the impurity peak of hydrochlorothiazide was eluted simultaneously with the hydrochlorothiazide peak. The amlodipine peak was eluted at 4.849 min, achieving purity, tailing factor, and theoretical plate number, however the capacity factor was still low (K = 0.8). We can see that telmisartan only appeared a peak in the chromatogram at 9.676 min and reached all chromatographic parameters.
In chromatographic condition 4, the hydrochlorothiazide peak has an increased retention time compared to that in chromatographic condition 3, from 3.656 min to 4.531 min, reaching purity (separable with impurity peak of hydrochlorothiazide - impurity peak appears at 4.160 min – resolution coefficient RS =2.3), capacity factor improved but still not reached (from 0.4 to 0.7). The amlodipine peak was eluted more slowly with a retention time from 4.849 min to 13.933 min, the capacity factor increased (from 0.8 to 4.3) and achieved other chromatographic parameters. The telmisartan peak appeared at 33.506 min with high-capacity factor (K = 11.7).
Therefore, we continue to conduct chromatographic investigation of the mixed standard solution at the ratio of 40:60 and 30:70 but replace acetonitrile with methanol (chromatographic condition 5). In this condition, the hydrochlorothiazide peak did not change retention time significantly (from 4.531 min → 4.132 min), however, the amlodipine peak eluted much more slowly (46.666 min) and did not appear telmisartan peak after chromatographic time 60 min. At the same time, with this mobile phase ratio, the pressure of the column increases (2950 psi), affecting badly to the column.
In order to improve the chromatographic parameters, especially the capacity factor of the three active peaks, and separate the hydrochlorothiazide peak and its impurity peak, a gradient elution program with gradually reduced polar mobile phase ratio is selected as an alternative to isocratic elution for further investigation. When changing to the gradient elution program (1) (Table 2), compared with the isocratic ratio of 30:70, the retention time of the hydrochlorothiazide peak was not significantly reduced (4.531 min → 4.488 min), the number of theoretical plates increased slightly (from N=9994 → N=10638), and the hydrochlorothiazide peak separates from its impurity peak with a resolution coefficient RS=2.1. Retention time was significantly reduced for amlodipine peak (from 13.933 min → 8.983 min) and telmisartan peak (33.506 min →13.993); however, the retention time of the telmisartan peak is still relatively long (13.993 min).
| Time (minutes) | Acetonitrile (% v/v) | Phosphate buffer pH 3.0 (% v/v) |
|---|---|---|
| 0 | 30 | 70 |
| 8 | 40 | 60 |
| 15 | 40 | 60 |
| 18 | 30 | 70 |
To improve this, we change to investigate with gradient elution program (2) (Table 3): The hydrochlorothiazide, amlodipine and telmisartan peaks were completely separated and chromatographically pure. The chromatographic parameters of the peaks met the stated requirements. The analysis time was just 15 min.
| Time (minutes) | Acetonitrile (% v/v) | Phosphate buffer pH 3.0 (% v/v) |
|---|---|---|
| 0 | 25 | 75 |
| 8 | 50 | 50 |
| 15 | 25 | 75 |
In this study, we choose wavelength 230 nm to observe the peaks in the chromatograms because 230 nm is the intermediate wavelength between the two maximum absorption wavelenths of hydrochlorothiazide (224.5 nm) and amlodipine (237 nm).
Thus, after the investigation process, we choose the mobile phase ACN - ammonium dihydrophosphate buffer with gradient program (2) to simultaneously quantify amlodipine, telmisartan, hydrochlorothiazide. (Figure 1)
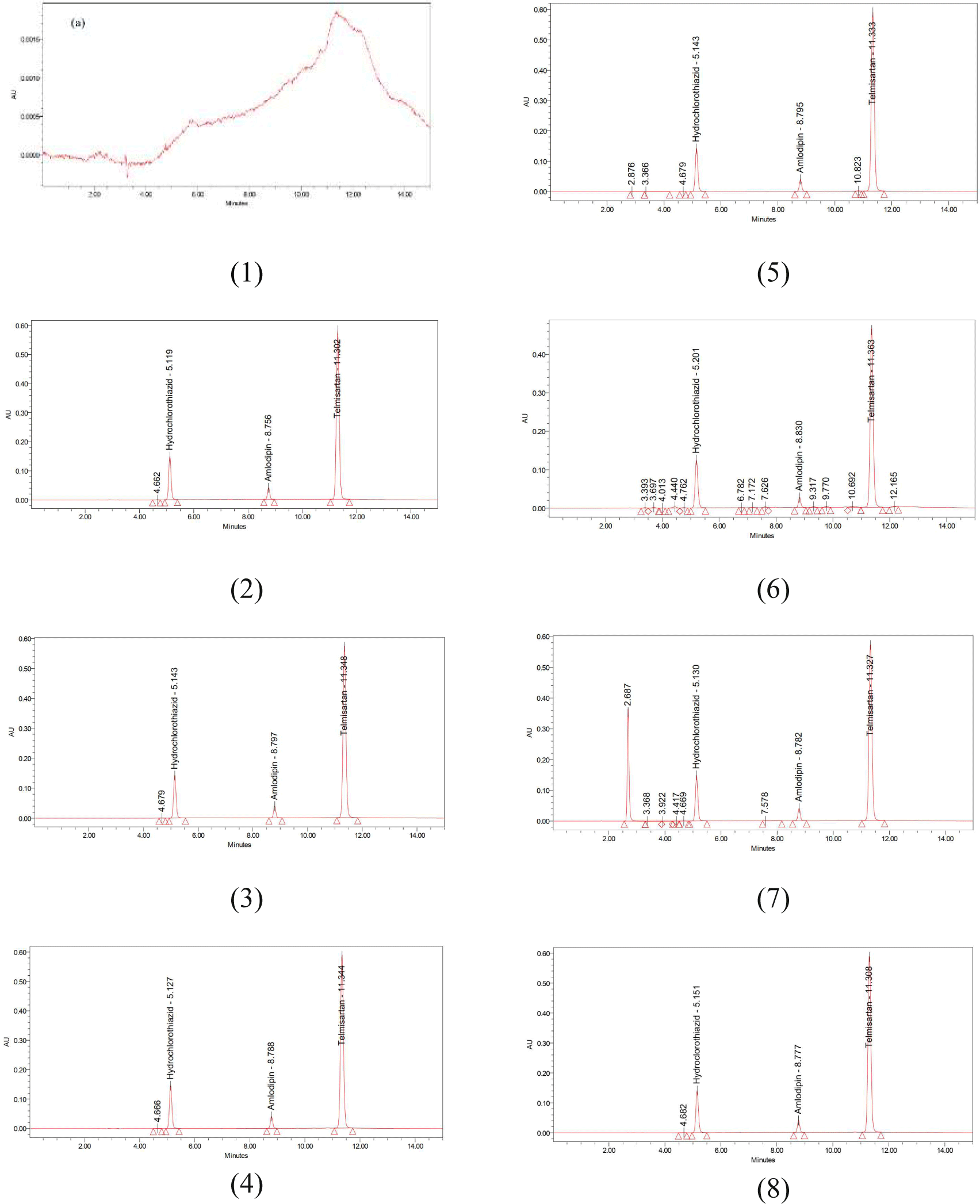
When conducting the specificity of the procedure, it was found that in the chromatograms of the test samples, there were peaks with retention times corresponding to those of hydrochlorothiazide, amlodipine and telmisartan in the chromatograms of the standard mixture solution. In the chromatograms of the degradation samples, three peaks of hydrochlorothiazide, amlodipine and telmisartan appeared at retention time corresponding to the retention time of the principal peaks in the chromatograms of the standard mixture solution. These peaks are separated completely and separated from other impurity peaks and obtained purity.
The validation results (Figure 2 and Table 4) showed that the procedure for simultaneous quantification of hydrochlorothiazide, amlodipine besylate and telmisartan by the HPLC-PDA method met all the requirements about:
-
System suitability (RSD% value of retention time and peak area ≤ 2%, resolution between peaks ≥ 1.5, tailing factor between 0.8 – 1.5 and theoretical plate number of peaks more than 2000).
-
Specificity (peaks were clearly separated, having purity (Figure 2), impurity peaks generated under extreme conditions do not coincide with the principal peaks).
-
Linearity: correlation coefficient R > 0.999, linear concentration range of HCT is 12.52 – 37.55, of AML is 5.00 - 15.01, and TEL is 40.01 – 120.02 ppm. The linearity of this procedure is similar to that reported in the references.
-
Accuracy: the recovery rates of all three substances were in the range of 98-102%.
-
Precision: includes repeatability and intermediate precision with RSD values no more than 2%.
-
Robustness (system suitability of standard mixture solution with suitable chromatographic parameters when varying the pH of the buffer solution from 2.8 to 3.2).
4. DISCUSSION
In reverse phase chromatography, the ability to separate substances depends on many factors such as the nature of the analyte, the type of stationary phase, the nature of the mobile phase, the ratio of the mobile phase, etc. Depending on the nature of the analytes for which we needs to select the appropriate mobile phase based on the results of experimental investigations or from reference documents. Initially, based on published quantitative articles on amlodipine besylate, telmisartan and hydrochlorothiazide, as well as monographs of these active substances in Vietnamese pharmacopoeia V and USP-NF 2021, the mobile phase was selected as acetonitrile – phosphoric acid water 0.01% (apparent pH 3.0). Current chromatographic columns still contain acidic free -Si-OH radicals (except for some kinds of column using end-capping techniques), especially the ionized form -Si-O-that will interact with analytes, affecting the peak shape as well as retention time, so when acidifying the mobile phase, it will limit the ionized free silanol radicals causing bad interaction with the analytes. The investigation results of the mobile phase (acetonitrile - phosphoric acid water 0.01%) showed that when increasing the percentage of phosphoric acid by 0.01%, the corresponding retention time of the three active peaks increased. This is explained by the fact that as the proportion of polar solvents in the mobile phase increases, the distribution of analytes between the stationary and mobile phases will be changed, therefore the analytes will increase interaction with the stationary phase so they were eluted from the chromatographic column later, resulting in a corresponding increase in the retention times of the active substances.
Hydrochlorothiazide is a fairly polar compound, logP= – 0.58; at the same time, hydrochlorothiazide has a pKa = 9.09 value, in the acidic mobile phase at pH 3.0, applying the Henderson - Hasselbalch equation for the secondary amine group shows that hydrochlorothiazide mainly exists in the ionized form so this substance interacts more with the polar mobile phase and less with the stationary phase. Amlodipine besylate is the salt of benzenesulfonic acid with amlodipine, which is generally polar. Amlodipine base has pKa = 8.6, in acidic mobile phase at pH 3.0, application of Henderson - Hasselbalch equation for primary amine functional group shows that amlodipine mainly exists in ionized form, should increase ability to interact with the polar mobile phase. However, amlodipine has a logP = 0.65 value, and is therefore less polar than hydrochlorothiazide (logP = -0.58), which explains the elution order of these two peaks in the chromatograms. Finally, telmisartan is the least polar compound with 2 benzimidazole groups and 2 lipophilic benzene rings in the structure, so this compound interacts strongly with the stationary phase. Telmisartan has three pKa values of 3.5, 4.1, and 6.0, respectively, for the carboxylic functional group and the two benzimidazole rings in the formula. In an acidic mobile phase environment (pH 3.0), applying the Henderson – Hasselbalch equation to the carboxylic functional group shows that the COOH form exists 3.16 times more than the COO-form. This could explain the fact that in the mobile phase acetonitrile - phosphoric acid water 0.01% (pH 3.0), telmisartan may exist in many different molecular and ionic forms, leading to the appearance of peaks at different retention times. The mobile phase is acidic water which is unable to maintain pH, so we should use other buffers to increase the stability of the mobile phase. We reference the monographs of the three main active ingredients in USP-NF 2021, continue to investigate with pH 3.0 buffer, and as recommended by the Xterra column, the appropriate buffer at pH 3.0 is the buffer of dihydrophosphate salts. Among the salts of dihydrophosphate, ammonium dihydrophosphate is chosen because this salt has better solubility in acetonitrile than other salts (sodium, potassium). When investigating the organic solvents of mobile phase: acetonitrile and methanol, we found that methanol eluted the active ingredients worse, prolonging the analysis time. At the same time, when using methanol, the high pressure of the column (about 3000 psi) reduces the life of the column and is not good for the pump of the system. Meanwhile, acetonitrile elutes the substances with more reasonable retention time, low column pressure (about 2000). Therefore, acetonitrile is the mobile phase solvent of choice.
On the other hand, through UV spectroscopy, a related impurity of hydrochlorothiazide appeared in chromatogram both the standard mixture and the test sample. For the quantification procedure to be specific, it is a prerequisite that the three major active peaks are completely separated from each other and from any impurity peaks if there were. Therefore, a gradient elution program in which the mobile phase with gradually decreasing polarization is needed as an alternative to isocratic run. The gradient program should include a change in solvent ratio over an appropriate period of time in order to separate the hydrochlorothiazide peak and related impurities of hydrochlorothiazide, and avoid that amlodipine and telmisartan peaks are eluted too slowly which leads to increase the analysis time of the process.
The detection wavelength was chosen to be 230 nm instead of 237 nm (which is the absorption maximum for amlodipine) in order to increase the signal response of hydrochlorothiazide to the probe because hydrochlorothiazide has an absorption maximum at 224.5 nm and an absorption minimum at the 237 nm region.
Robustness of procedure was validated when the pH change was performed. The investigation shows that the small change in pH does not affect the quantitative process, proving that the process has a certain stability.
Conclusion
A procedure for the simultaneous quantification of amlodipine besylate, telmisartan and hydrochlorothiazide in the preparation has been developed by high-performance liquid chromatography. The procedure was validated and met for system suitability, specificity, linearity, accuracy, precision, working range and robustness.









