1. INTRODUCTION
Fetal growth restriction (FGR) refers to the incapacity of the fetus to reach its potential expected growth due to pathological reasons, with placental malfunction being the most common [1]. FGR is accompanied by an enhanced risk of adverse perinatal outcomes (APO), such as fetal death, perinatal morbidity and mortality [2].
There are two distinct phenotypes of FGR, which are classified by gestational age at the time of diagnosis: early-onset FGR (<32 weeks) and late-onset FGR (≥32 weeks) [3]. Nowadays, there is an international guideline from the Trial of Randomized Umbilical and Fetal Flow in Europe (TRUFFLE) Study stage 1 about the diagnosis and management of early-onset FGR based on cCTG and ductus venosus to improve perinatal outcomes [4]. Nevertheless, there is no international consensus on identifying fetuses with late-onset FGR who are at high risk of compromise or on the optimal surveillance protocol or timing of delivery, with discordant guidance ranging from 37 to 38+6 weeks of gestation [5–7]. Without any effective treatment for FGR, the backbone of management of late-onset FGR is strict surveillance and appropriate delivery timing. Therefore, the identification of fetuses at risk of APO is critical to enhancing the possibility of better perinatal outcomes in pregnancies with late-onset FGR.
This study aims to evaluate the prevalence of APO in late-onset FGR, examining some clinical parameters, such as maternal characteristics, fetal biometrics (abdominal circumference [AC], estimated fetal weight [EFW]) and Doppler parameters (cerebroplacental ratio [CPR], umbilical artery PI [UA-PI], middle cerebral artery PI [MCA-PI]) to determine factors related to APO.
2. METHODS
This was a retrospective cross-sectional study in a single tertiary referral center (Tu Du Hospital, Ho Chi Minh City, Vietnam) and included all individuals diagnosed with late-onset FGR between April 1st, 2022, and December 31st, 2022.
FGR is defined based on criteria of Delphi Consensus, in which late-onset FGR was defined as “estimated fetal weight (EFW) or AC<3 percentile, or EFW or AC<10 percentile and UA pulsatility index (PI)>95 percentile or CPR<5 percentile or AC/EFW crossing percentile by >two quartiles on growth charts”, diagnosed after 32 weeks [8]. Severe late-onset FGR was defined as AC or EFW less than the 3rd percentile. The inclusion criteria were singleton pregnancies with late-onset FGR diagnosed at >32 weeks of pregnancy. Exclusion criteria were cases with FGR diagnosed before 32 weeks of pregnancy, chromosomal abnormality and/or congenital malformations, infection, or lack of routine serial ultrasounds during pregnancy. The study was performed following the STROBE guideline [9].
For Z2 (1–α / 2): critical value at 95% CI (Z [1–α / 2]=1.96, where α=0.05). Selected prevalence (p) was 28%, according to a study in Turkey by Kahramanoglu et al.; d=0.09. The minimum sample size was 95.6. Through medical records, there were 101 late-onset FGR pregnancies admitted to Tu Du Hospital in Ho Chi Minh City between April 1st, 2022, and December 31st, 2022. We retrospectively included all these late-onset FGR pregnancies.
Maternal characteristics and clinical data were collected from medical records and ultrasound databases. Maternal age, previous maternal pathology and/or obstetrical pathology, parity, pregestational body mass index (BMI), gestational age at diagnosis, gestational age at diagnosis, and gestational age at delivery of late-onset FGR were evaluated. Maternal age was calculated as the current year minus the patient’s year of birth. Pregestational BMI was calculated based on weight and height before pregnancy and grouped based on the Asia-Pacific classification of BMI. Gestational age was determined by comparison of the last menstrual period and the first-trimester crown-rump length measurement according to the International Society of Ultrasound in Obstetrics & Gynecology (ISUOG) guidelines. At the Radiology Department of Tu Du Hospital, Doppler records were performed by Samsung HS40, HS60, Hera W10, and R7 ultrasound device (Samsung Medison Company, Suwon, Korea) with a 1–8 MHz volumetric probe, which has color and spectral Doppler functions. In the last fetal ultrasound, biometric measurements (EFW, AC, and single deepest vertical pocket [SDP]) and fetal Doppler measurements (UA-PI, MCA-PI, CPR) were assessed. EFW was calculated using the Hadlock-3 formula [10]. Doppler indexes were recorded following to the recommendations of ISUOG [11]. EFW, AC, and Doppler parameters were referred to The Fetal Medicine Foundation (FMF) standard to determine the percentile [12]. Doppler indices were considered normal between the 5th percentile and 95th percentile. Doppler indices were considered abnormal when they were less than the 5th percentile (MCA-PI, CPR) or more than the 95th percentile (UA-PI). Oligohydramnios was defined as a vertical pocket of amniotic fluid less than 2 cm, while polyhydramnios was defined as SDP greater than 8 cm. The determination of the time and the mode of delivery was based on stages of FGR. The monitoring interval was based on stages of FGR. Fetal growth was assessed every 3 weeks.
In newborn records, 5-minute Apgar score, birth weight, gestational age at delivery, the need for admission to the neonatal intensive care unit (NICU), overall length of hospital stay, respiratory distress requiring mechanical ventilation (non-invasive or invasive ventilation), neonatal jaundice requiring phototherapy or blood exchange, neonatal hypoglycemia, and perinatal mortality were recorded. The fundamentals of the Apgar score include color, heart rate, reflexes, muscle tone, and respiration. At Tu Du Hospital, the Apgar score was recorded in all newborn infants by nurses in theDelivery Department unless the infants required pediatrician consultation indication at the delivery. Neonatal hypoglycemia was defined by a capillary glucose measurement of <2 mmol/litre [13]. Perinatal mortality was the death of a fetus from 22 weeks of gestation to 7 days after birth [14]. APO were defined as the presence of at least one of the following: 5-minute Apgar score<7, NICU admission, respiratory distress requiring mechanical ventilation, neonatal jaundice requiring phototherapy or blood exchange, neonatal hypoglycemia, or perinatal death.
Categorical data are presented as numbers and percentage, while continuous variables are presented as the mean±SD. In our study, the data were entered into Microsoft Excel software version 365 MSO, and there was no missing data. Statistical analysis was performed using R 4.3.1 software (R Foundation for Statistical Computing; https://www.r-project.org/). The prevalence of APO in women diagnosed with late-onset FGR was calculated. In the univariable analysis, comparisons between pregnancies without APO and those with APO were performed using the χ2 test for categorical variables, whilst comparisons in continuous variables were analyzed using the Student’s t-test. Variables with a p-value<0.2 were analyzed with a multivariable logistic regression model to examine the power of the association between maternal characteristics of pregnancies and ultrasound parameters with APO. The results from the multivariable logistic regression analysis are reported as adjusted odds ratios (aOR) with 95% confidence interval (CI). A p-value<0.05 was considered to manifest statistical significance.
3. RESULTS
Through the electronic database system, from April 1st, 2022, and December 31st, 2022, a total of 589 medical records were evaluated. A total of 101 pregnant women with suspected late-onset FGR were recruited based on inclusion criteria and exclusion criteria (Fig. 1). Among these 101 pregnant women, 80 newborns went to the nursery, and 21 newborns were admitted to the NICU.
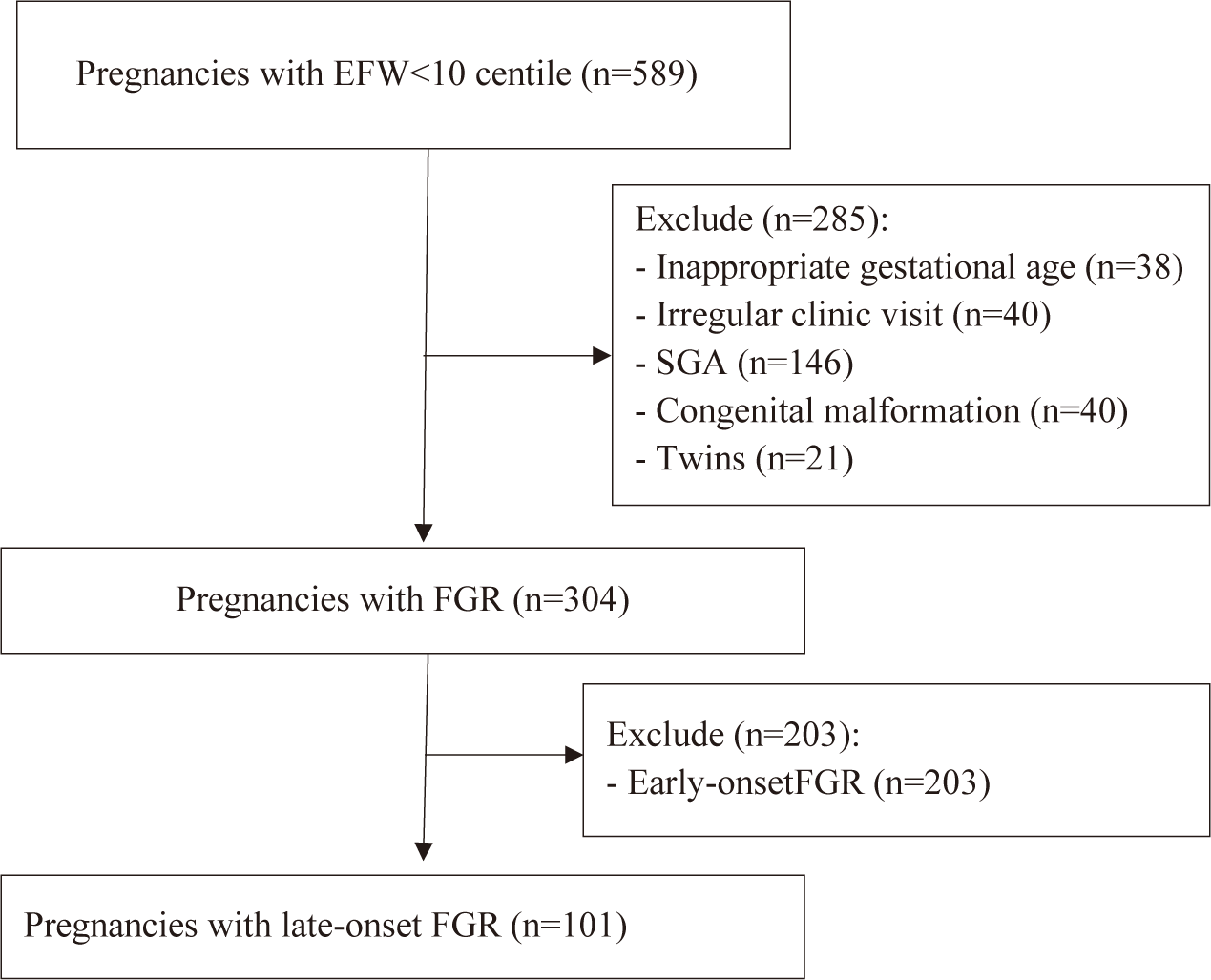
By the end, our study recruited 101 participants with the following characteristics:
The socio-economic characteristics of participants are presented in Table 1. Most of the participants were in the reproductive age range of 18–35 years old. Almost all patients lived outside Ho Chi Minh City (63.4%). The majority of participants’ occupations were office staff (36.6%), followed by housewife (31.7%), worker (19.8%), trader (9.9%), and farmer (2.0%).
The adverse outcome occurred in 21 (20.8%) infants, and the prevalence was demonstrated in the table below. The need for admission to the NICU, mean overall length of hospital stay, respiratory distress requiring mechanical ventilation, neonatal jaundice requiring phototherapy, and neonatal hypoglycemia were recorded in 21 (20.8%), 6.67 days, 11 (10.9%), and 2 (2%) cases, respectively, while no case of perinatal death or 5-min Apgar score<7 was recorded in the study (Table 2).
The maternal characteristics of the pregnancies with late-onset FGR were described in Table 3. The mean birthweight (2,545.12±268.74 g vs. 2,245.24±302.04 g, p<0.001), gestational age at birth (38.1±1.01 vs. 36.6±1.2, p<0.001), and gestational age at diagnosis (37.6±1.12 vs. 36.6±1.2, p=0.004) were lower in the fetuses who suffered APO compared to the control group. Meanwhile, there was no difference in terms of maternal age, chronic diseases before pregnancy, nulliparity, gestational diabetes, hypertensive diseases, delivery mode, or BMI between the two study groups (p>0.05).
In Table 4, there was a significant difference in the parameters of CPR<5th percentile (p=0.016) and EFW<3rd percentile (p=0.046) in predicting APO, while there was no significant difference in the UA-PI>95th percentile (p=0.209), MCA-PI<5th percentile (p=0.758), AC<3rd percentile (p=0.61), or oligohydramnios (p=0.99) in the APO group compared to the control group in late-onset FGR pregnancies (p>0.05).
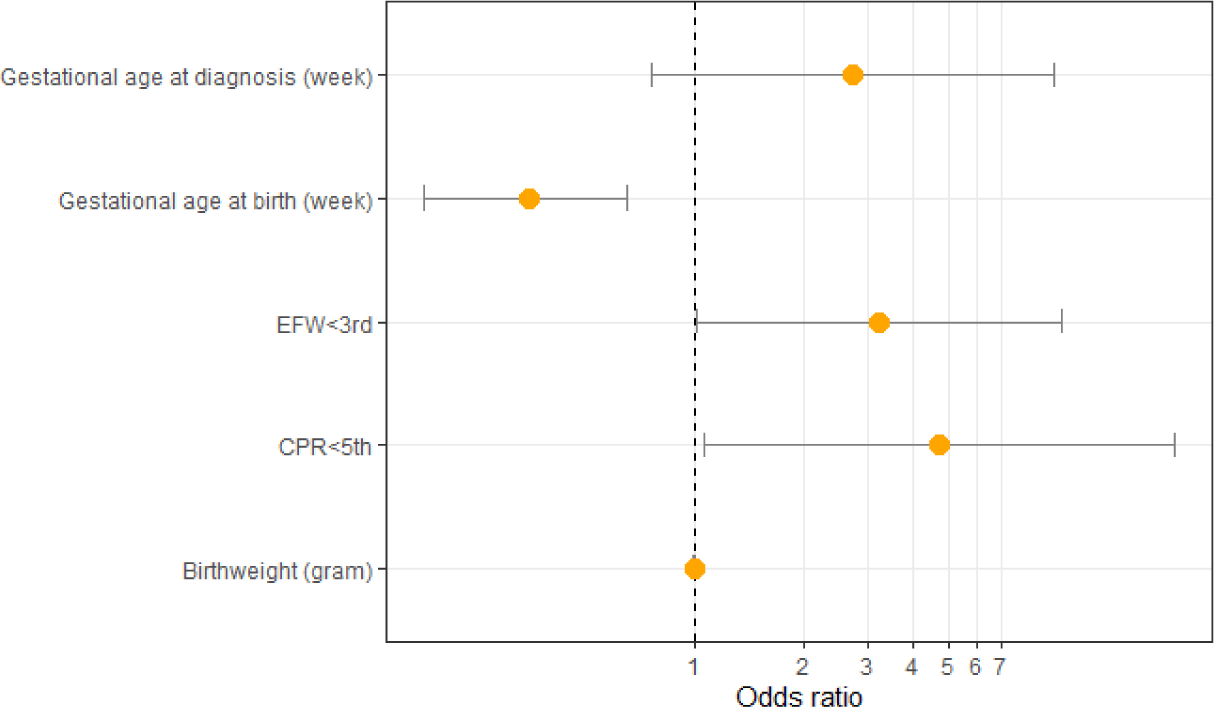
After univariate analysis, there are five factors with p-value<0.2: CPR<5 percentile, EFW<3 percentile, gestational age at delivery, gestational age at diagnosis, and birthweight. All five of these factors were analyzed with the multivariate logistic regression model. After multivariate logistic regression analysis, there are three factors that are actually related to the APO of pregnant women with late-onset FGR: CPR<5th percentile (adjusted odds ratio [aOR]=4.76, 95% CI=1.07–21.11, p=0.04), EFW<3 percentile (aOR=3.22, 95% CI=1.01–10.27, p=0.049), and gestational age at birth (aOR=0.35, 95% CI=0.18–0.65, p=0.001) (Table 5 and Fig. 2).
4. DISCUSSION
Our research demonstrated that about 20.8% of pregnancies complicated by late-onset FGR experienced APO. The prevalence of APO in pregnancies with late-onset FGR in our research was consistent with that in previously published literature, affirming the high rate of APO in these pregnancies [15–20].
When investigating the various Doppler parameters, the percentage of CPR<5th percentile and EFW<3rd percentile were higher in pregnancies that had APO compared with those that did not, whilst there was no difference in the percentage of UA-PI>95th percentile, MCA-PI<5th percentile, AC<3rd percentile, or oligohydramnios between the study groups. In addition, compared with pregnant women without APO, the mean birthweight, gestational age at birth, and gestational age at diagnosis were lower in the fetuses who suffered APO. On multivariable logistic regression analysis, CPR<5th percentile, EFW<3rd percentile, and gestational age at delivery were independently and statistically associated with APO (p<0.05).
Our study demonstrated that in severe fetal late-onset FGR, EFW<3rd percentile conferred a 3.22-fold increased risk of composite APO (aOR=3.22, 95% CI=1.01–10.27, p<0.05). The value of EFW<3rd percentile was equivalent to that reported in the literature [21–24]. In cohort research conducted by Savchev et al. [25] 132 term small-for-gestational age (SGA) fetuses with Doppler parameters were compared to a control group of 132 appropriate-for-gestational-age (AGA) fetuses. The result demonstrated that among SGA, fetuses with EFW<3rd percentile had a higher risk for APO compared to SGA fetuses with EFW≥3rd and the AGA group [25]. Meler et al. [24] performed a meta-analysis to evaluate the association between severe smallness and APO among late-onset SGA. The study included twelve cohort studies and a total of 3,639 fetuses with suspected late-onset SGA, of which 1,246 had suspected severe SGA. The results showed that there was a significant association between severe SGA and composite APO (OR=1.97, 95% CI 1.33–2.92, p<0.05), NICU admission (OR=2.87, 95% CI 1.84–4.47, p<0.05) and perinatal death (OR, 4.26, 95% CI 1.07–16.93, p<0.05) [24].
Among Doppler parameters, CPR was the only one that showed a strong association with APO. Our findings were also consistent with previous studies. Unlike early-onset FGR, whose pathophysiology is related to the abnormal transformation of the spiral arteries and placental insufficiency [26], the late-onset FGR is characterized by placental damage but with milder placental lesions [27,28]. In Doppler ultrasound, the blood flow of the UA depicts the proportion of fetal–placental perfusion. However, alterations in UA Doppler are uncommon and fail to detect the majority of late-onset FGR cases because the UA Doppler becomes abnormal only when a moderate portion of the placenta is damaged [27]. Most adverse events resulting from placental insufficiency in late-onset FGR occur without abnormal UA flow [29]. Cerebral vasodilation, through an adaptive mechanism called the brain-sparing effect, occurs when the fetus faces hypoxemia [30]. Cerebral vasodilation can be determined by measuring the MCA flow (MCA-PI) or the CPR. The reason for using the MCA-PI and UA-PI ratios, also known as the CPR, is that CPR can detect abnormalities and reveal the placental insufficiency that may not be apparent through the evaluation of each parameter.
In our findings, the investigation of cerebral Doppler measurements was consistent with previous studies, affirming that CPR becomes abnormal earlier than the MCA-PI [31]. Among SGA fetuses, the CPR has improved sensitivity for detecting adverse outcomes, such as perinatal mortality and admission to NICU, compared to either the UA or the MCA Doppler alone [30,32,33]. Studies have demonstrated that 15% to 20% of late-onset FGR fetuses with normal UA-PI had MCA Doppler findings of cerebral vasodilation, and CPR has been highlighted for its value in predicting APO and guiding the timing of delivery in late-onset FGR [15,16,23,34–36]. A study by Flood et al. [33] resulting from the multicenter Prospective Observational Trial to Optimise Paediatric Health in IUGR (PORTO), showed that, in late-onset FGR diagnosed after 34 weeks of gestation, CPR PI less than 1 increased the OR of APO to 10.7 (95% CI, 2.4–48.7; p<0.05) [36]. To date, there was an international consensus about delivery in fetuses with EFW<3 percentile at 37 weeks of gestation. However, no international consensus exist on the delivery timing of a late-onset FGR fetus with signs of cerebral blood distribution and optimal timing of delivery based on abnormal CPR, with discordant guidance ranging from 37 to 38+6 weeks of gestation [5–7]. The TRUFFE-2 study was a randomized controlled trial involving 12 UK as well as European and international centers. The objective of TRUFFE-2 was to test the hypothesis that delivery based on cerebral blood flow redistribution can reduce a composite of perinatal and long-term outcomes. This study officially started in December 2019, and the results are expectedted to be published in the next few years.
Through multivariate association using the logistic regression model, gestational age at delivery also contributed to APO (aOR=0.35, 95% CI=0.18–0.65, p=0.001). The lower gestational age at delivery increased the likelyhood of neonates suffered from APO. Our finding about the association between gestational age at delivery and APO is consistent with previous literature, affirming the high rate of APO in the preterm population [37–39] and preterm FGR [40].
First and foremost, our study was a retrospective cross-sectional study. Therefore, factors related to APO in late-onset FGR identified by multivariate regression analysis can only indicate a statistically significant association. Since our research was conducted within a limited timeframe, our sample size was relatively small compared to other studies with identical topics. Furthermore, our research samples were collected solely from Tu Du Hospital rather than from multiple centers. For that reason, our results may not be representative of all pregnant women in Vietnam and could be subject to selection bias.
5. CONCLUSION
Late-onset FGR is associated with an increased risk of APO. Currently, there is no effective treatment for FGR. Therefore, the recognition of fetuses with signs of perinatal compromise is crucial to minimizing APO in late-onset FGR pregnancies. In conclusion, our research demonstrated that CPR<5 percentile, severe late-onset FGR, and gestational age at delivery were independently and statistically associated with APO.









