1. INTRODUCTION
Mitral regurgitation (MR) affects 2%–3% of the global population [1]. The incidence of MR caused by degenerative factors is rising in developing countries, while rheumatic valve disease remains the primary cause of MR [1],[2]. One study in Vietnam shows there is a decrease in rheumatic mitral valve surgery from 95% to 81%, and an increase in the number of surgeries due to degenerative causes from 1.6% to 16%, and in the rate of surgery for degenerative MR from 11.4% to 38.5% [3].
The degenerative mitral valve pathomechanism affects the posterior leaflet or annulus and can typically be repaired through conventional repair techniques. Posterior leaflet prolapse can be corrected through leaflet resection or artificial chordae implantation while correcting the anterior or bi-leaflet prolapse requires more complex procedures and strategies. We aim to examine the safety and feasibility of minimally invasive mitral repair using artificial chordae in intermediate-to-complex valve regurgitation. Our goal is to determine whether such operations can be effectively applied, despite the challenges posed by complex valve pathologies.
2. MATERIALS AND METHODS
A retrospective analysis was performed on patients who underwent minimally invasive mitral valve repair using artificial chordae via right mini-thoracotomy at one center in Vietnam between April 2016 and April 2022. The indication for mitral valve surgery followed the current American College of Cardiology/American Heart Association (ACC/AHA) guidelines for the management of valvular heart disease [4]. A single, primary, cardiovascular surgeon, who had overcome the learning curve of mitral valve repair [5], and supported by his assistants, performed all the operations. The selection of the patient population was based on the Anyanwu complexity score. Intermediate and complex repair cases were selected. Furthermore, demographic information, echocardiographic findings, operative characteristics, surgical outcomes, and mid-term survival rates of the patients were collected and analyzed.
The Anyanwu complexity score [6] was used to categorize the valve repair into three levels of complexity: simple (score≥1), intermediate (score≥2–4), and complex (score≥5). Residual MR was defined as the MR of more than moderate severity at the time of transferring the patient from the operating room. Recurrent MR was defined as the occurrence of MR after an initial absence of the condition at the time of discharge from the operating room.
The data was analyzed using Microsoft Excel 2016 and SPSS version 16.0. Descriptive analysis was used to analyze the patient’s demographic and clinical characteristics and presented as mean±SD for continuous variables and frequency and percentage for categorical variables. Survival, freedom from reoperation, and recurrent MR were evaluated using Kaplan-Meier analysis.
3. RESULTS
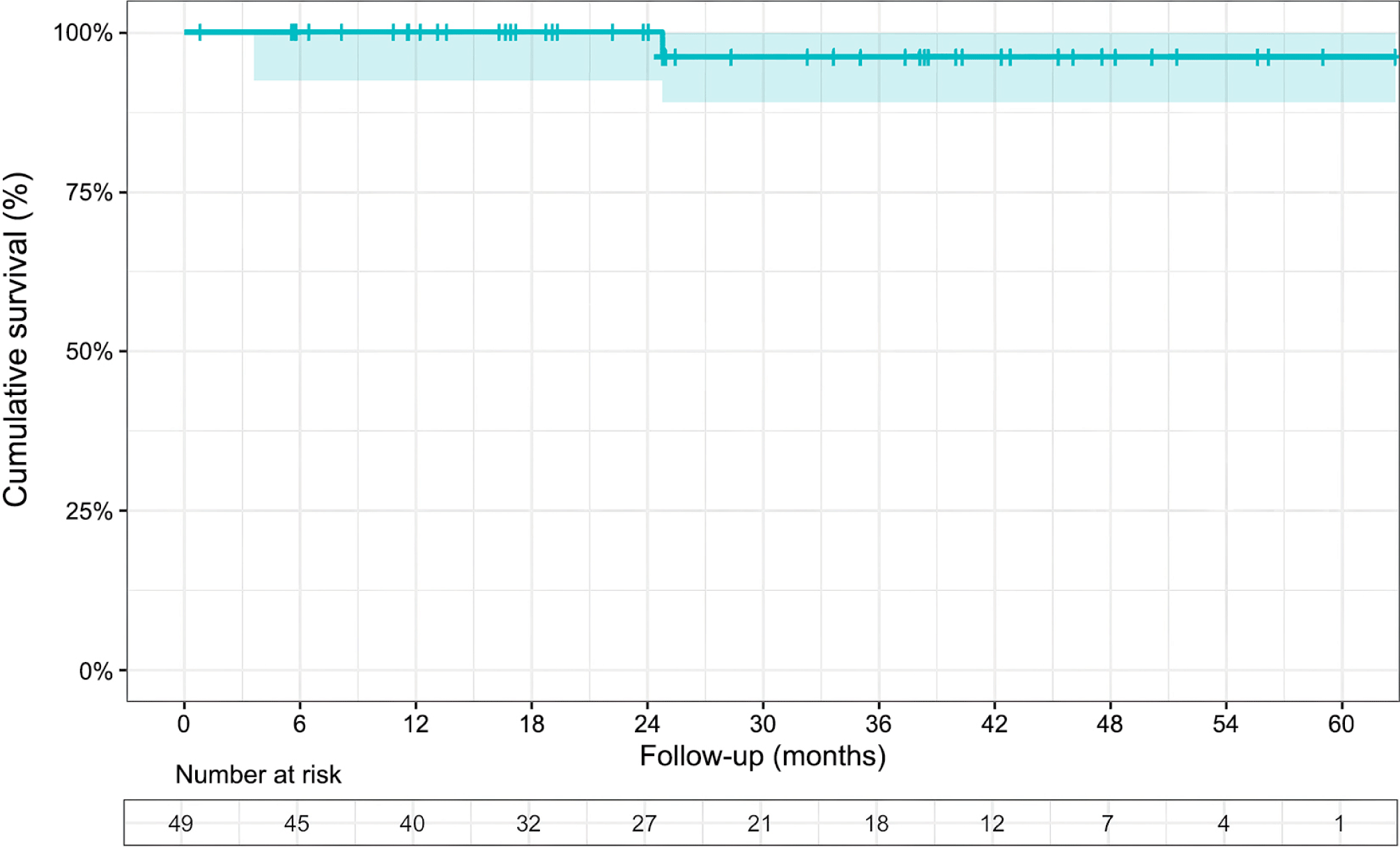
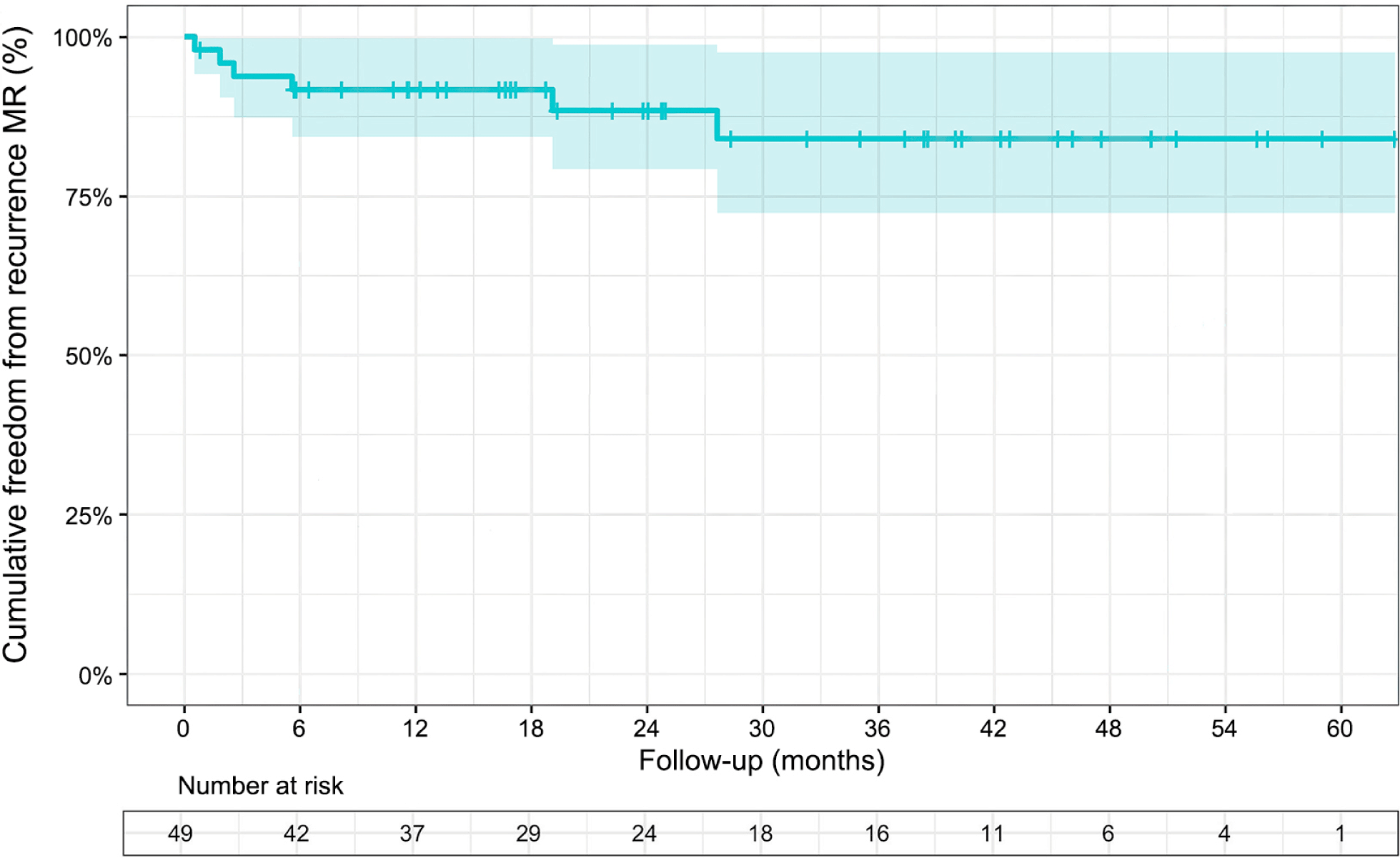
49 patients with intermediate-to-complex degenerative MR underwent minimally invasive mitral valve repair using artificial chordae via right mini-thoracotomy. The mean age was 49.1±13.3 years, with a male-to-female ratio of 3.1:1. The mean EuroSCORE II was 1.12±0.99%. All cases of MR in the study were classified as severe and categorized as Carpentier’s functional classification type II, with no cases of moderate-to-severe tricuspid regurgitation requiring repair observed (Tables 1–4, Figs. 1 and 2).
BMI, body mass index; BSA, body surface area; NYHA, New York Heart Association; LVEDD, left ventricular end-diastolic diameter; LVESD, left ventricular end-systolic diameter; LA, left atrial; LVEF, left ventricular ejection fraction; TAPSE, tricuspid annular plane systolic excursion; sPAP, systolic pulmonary artery pressure; A, anterior; P, posterior; FED, fibroelastic deficiency.
| Mid-term outcome | Value (N=49 [n]) |
|---|---|
| Overall mortality | 1 (2.0) |
| Mitral reoperation | 0 (0.0) |
| Follow-up mitral re-regurgitation | |
| None/trivial | 43 (87.8) |
| Moderate to severe | 6 (12.2) |
4. DISCUSSION
The current guidelines on mitral valve surgery emphasize repairing the valve instead of replacing it when possible [4]. The feasibility of repair, which is highly affected by the intricacy of the lesion and the surgeon’s expertise, plays a role in determining if surgery is needed.
To ensure a successful MR repair and assess its complexity, it’s crucial to use a proper grading method to assist in choosing appropriate techniques. Consultation with experts is encouraged, if needed. Surgeon experience is commonly assessed based on his years in practice. Li et al. [7] found that it generally takes between 50 to 200 operations for surgeons to surpass the learning curve associated with mitral valve repair rates, with some variation among individual surgeons. In our previous publication, when the primary surgeon has successfully surpassed the lower threshold of 90 operations, he has overcome the learning curve for mitral repair rates, thus enabling him to consider repairing more complex mitral valve lesions [5]. In general, the complexity of a mitral valve repair is considered simple if only the posterior leaflet is prolapsed; and is complex if the anterior leaflet or both leaflets are prolapsed [8]. Different authors have evaluated the complexity of mitral valve repair through various grading scores [9],[10]. However, Anyanwu’s system, based on echocardiography features, the location of leaflet prolapse, valve tissue calcification, and limited leaflet mobility is widely considered practical and comprehensive [6]. In our study, we used the complexity score of Anyanwu, as it has also been utilized by Nakayama [11].
Eleven patients (22.4%) were diagnosed with Barlow’s disease, a complex condition characterized by excessive myxomatous tissue in the leaflets, bi-leaflet prolapse, and severe annular dilation, which posed significant challenges. Four patients (8.2%) presented with complex valvular lesions based on Anyanwu’s grade. All these complex forms of mitral valves were repaired using a combination of ring annuloplasty and other repair techniques in which artificial chordae were used as the principal technique. Another important aspect of Barlow valve repair is mitral annular disjunction, a condition characterized by a separation between the atrial-mitral valve junction and ventricular attachment [4]. This can be observed during echocardiography video perioperatively. In these cases, the mitral annulus may have a flatter and more elliptical shape, with reduced height and loss of the saddle shape. Therefore, when implanting an annular mitral ring, using a complete rigid ring with a saddle shape is crucial to provide a more favorable and uniform distribution of forces, reducing strain on the mitral leaflet and improving leaflet coaptation geometry [12]. According to Rankin et al. [13], almost all patients with mitral disease can be effectively treated using artificial chord repair, even in complex cases. Our results showed that none of the patients had residual or recurrent MR at discharge. Nine patients (18.4%) had moderate MR according to postoperative echocardiography, and there was no conversion to valve replacement.
In the past, the technique for repairing posterior leaflet prolapse involved making a triangular or quadrangular resection on the posterior leaflet, followed by leaflet plication. Sliding-plasty techniques can reduce the risk of Systolic Anterior Motion (SAM) while ensuring the repair is effective [14], but may result in decreased mobility of the posterior leaflet. To overcome this issue, there has been a trend towards using artificial chordae for repairing extensive or multiple segments of prolapse of the posterior leaflet, especially in the P2 scallop. This procedure is considered more straightforward and allows for adjusting the height of the posterior leaflet, reducing the risk of SAM [15].
For repairing anterior leaflet prolapse or bi-leaflet prolapse, there is a general consensus among surgeons to use artificial chordae for all anterior prolapses. We would like to emphasize that bi-leaflet prolapse is a complex condition to repair. Castillo et al. [16] proposed a two-step process: first, repairing the posterior leaflet, followed by inserting a ring annuloplasty, and then conducting a saline test to evaluate the regurgitation state. Most injuries, including complex lesions, can be thoroughly evaluated at this stage, and various valve repair techniques, such as artificial chordae, commissurotomy, and edge-to-edge stitch, could be applied. According to Koprivanac et al. [17], in bi-leaflet prolapse, an annuloplasty ring is appropriate for repair if the prolapse is large and even, and neo-chordae implantation is suitable if the prolapse is asymmetrical.
In five cases (10.2%), we employed the Edge-to-Edge technique as a backup solution in case of an inadequate repair. This method is favored due to its transparency and efficiency, especially in challenging situations. The surgical procedure involved stitching the loose edges of the leaflets at the site of regurgitation, resulting in a valve with two orifices when the regurgitation originates from the middle folds, as described by Alfieri et al. [18].
We employed commissuroplasty in 13 cases (26.5%). Although less common than anterior and posterior leaflet prolapse, comissural prolapse is not uncommon and often occurs as residual lesions following the primary repair. Diagnosis can be challenging, as it may be missed in one-third of cases and difficult to detect through preoperative echocardiography [19]. The typical repair method is commissural closure, which does not result in significant flow restriction [19]. In addition, 11 patients (22.5%) underwent interscallop indentation closures. Indentation can develop as the leaflet volume expands, which can be easily identified through a saline test and can be easily repaired with a single stitch.
Our surgical results showed a mean aortic clamp time of 92.6±20.6 min and a mean cardiopulmonary bypass (CPB) time of 201.1±67.7 min, which were longer than the results achieved by Nakayama [11]. The author repaired 141 cases of MR using a mini-invasive approach via right thoracotomy (81% classified as intermediate to complex), and outcomes showed a CPB time of 144±36 min and a cross-clamp time of 113±35 min [11].
There were no in-hospital deaths in our study. During the postoperative period, one case of pleural effusion required re-exploration via the same thoracic incision. Another case of postoperative low cardiac output syndrome was recognized, caused by low blood pressure and a decline in left ventricular function. An intra-aortic balloon pump was inserted and successfully removed. The patient was then discharged from the hospital successfully. Nakayama did not encounter any cases of low cardiac syndrome in his record [11].
A case of postoperative lower limb compartment syndrome was recorded in a patient who underwent peripheral CPB with a 22-Fr femoral artery cannula, leading to an extended CPB duration of 216 min. The syndrome was diagnosed 10 h post-surgery and presented symptoms of severe pain, coldness, tenderness, paresthesia, and weak peripheral pulses. The limb was saved with a fasciotomy. The patient’s high body mass index, large CPB cannula size, extended operation time, and Kawashima’s Type D vasculature [20] were identified as risk factors. The incidence of limb compartment syndrome was extremely low. Out of 2,645 patients who underwent minimally invasive cardiac surgery, only two cases of lower extremity compartment syndrome (0.08%) were documented [21]. The rareness and uniqueness of this patient’s situation have been reported in our previous case report publication [22].
In this study, freedom from recurrence of MR was 92% at 12-mon, 88% at 24-mon, and 84% at 36-mon. These results suggest a low incidence of recurrent MR but inferior to those found in the outcomes of Castillo et al. [16], which involved 188 patients with degenerative mitral valve disease and either isolated anterior leaflet or bi-leaflet prolapse. Research by David et al. [23] indicates that early recurrent MR is often caused by technical errors or insufficient repair, while late recurrent MR is mainly due to degenerative progression. During follow-up, no reoperations due to MR recurrence were found in our study. According to Chitwood [24], mitral valve repairs can fail, despite being performed by skilled surgeons. The reasons behind these failures can be attributed to the progression of the disease, endocarditis, and technical issues such as the dehiscence of the prosthesis and the tearing of sutures.
Our results revealed that the survival rate measuring at 24-mon and 36-mon was 100% and 96%, respectively. Only one death was recorded during the follow-up. These results are considered favorable when compared to previous studies. For example, Nakayama reported 1-year and 3-year survival rates of 99.2% and 98.1%, respectively [11]. Castillo and his colleagues [16] found a cumulative survival rate of 96.3% at 4-year in patients in case of bi-leaflet prolapse. The author’s predominant repair technique was artificial chordae or loop in 49% of operations. Overall, these findings suggest that our results demonstrate a satisfactory level of patient survival compared to previous studies.
The current study has several limitations. Firstly, its retrospective design may introduce biases and limitations in data collection. Additionally, the relatively small sample size and single-center setting raise concerns about the generalizability of the findings. Another limitation is that the Anyanwu complexity classification used in the study may not be comprehensive enough as it did not consider certain factors such as the presence of mitral annular disjunction. Moreover, since the study was conducted by a single surgeon, there may be biases due to differences in preferred repair strategies compared to other surgeons. To address these limitations, future research should involve larger prospective studies conducted in multiple centers with different surgeons, using different complexity score systems to enhance the reliability of the evidence.
5. CONCLUSION
In conclusion, our study demonstrates that minimally invasive repair of complex mitral valves using artificial chordae is a feasible approach with low morbidity rates and recurrence of MR. The success of this repair technique depends on the accurate evaluation of the lesion’s complexity using proper grading system and perioperative valve analysis. By combining different repair techniques with the correct repair strategies, it could enhance the success rate of MR repair significantly.









