1. INTRODUCTION
Linezolid, an oxazolidinone antibiotic, works by inhibiting bacterial peptide synthesis through binding to the peptidyl transferase site of the 50S ribosome subunit. This mechanism allows linezolid to be effective against a wide range of multidrug-resistant Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant Enterococci, methicillin-resistant coagulase-negative Staphylococci, and penicillin-insensitive Streptococcus pneumoniae. Linezolid is licensed to treat a number of illnesses including bacteremia, nosocomial pneumonia, skin and soft tissue infections, and community-acquired pneumonia [1–5]. Linezolid is now used to treat a variety of difficult infections, including prosthetic infection, bone and joint infections, and multi-drug resistant tuberculosis, which require lengthy treatment durations [5–8]. However, this increased use has generated safety concerns, as the maximum recommended duration of treatment is limited to 28 days due to the potential for drug-related side events [5].
Linezolid has good pharmacokinetic characteristics, with a bioavailability of approximately 100%, allowing for interchangeable administration via oral or intravenous routes without dose adjustment. It mostly metabolized through the oxidation of its morpholine ring into an inactive form via non-enzymatic oxidative reactions, and the kidneys eliminate approximately 30% to 40% of the parent drug as an unchanged form in the urine. Additionally, linezolid has been labeled as a “one dose fits all’ treatment for patients with varying degrees of renal function [1–5]. Due to its advantages, linezolid has been widely used to treat Gram-positive infections.
However, various linezolid-induced side effects have surfaced in post-marketing surveillance, although they were documented with a low prevalence in controlled clinical trials. Among these, thrombocytopenia has been identified as one of the most severe adverse effects associated with linezolid treatment, with a prevalence ranging from 13.9% to 60.5%, depending on the definition used [9,10]. The specific mechanisms underlying linezolid-induced thrombocytopenia are unknown, however they may include platelet formation inhibition and increased elimination of platelets. Some publications suggest that linezolid suppresses the release of platelets from mature megakaryocytes by promoting myosin light chain 2 phosphorylation [11]. Sasaki et al. using a proliferation cell model, demonstrated that thrombocytopenia was due to inhibiting the proliferation of progenitor cells. Boak, using a stem cell model, discovered that a linezolid concentration of 8.06 mg/L led to a 50% reduction in the synthesis of platelet precursor cells [12,13]. Whereas, other mechanisms have been identified, such as immune-mediated platelet destruction [14,15] and nonimmune-mediated destruction, which resulted from reduced antioxidation capacity and the generation of lipid peroxidation and free radicals in patients who have been treated with linezolid for more than 20 days [16]. In clinical practice, thrombocytopenia can lead to the need for platelet transfusion and other support therapy, and its early onset may result in premature linezolid discontinuation, which could affect the selection of appropriate treatment regimens because linezolid is one of the best options for oral treatment for outpatients.
Even though the current Food and Drug Administration (FDA)-approved labeling for linezolid indicates that no dose adjustment is equired for patients with renal dysfunction, post-approval observational studies have reported a higher incidence of thrombocytopenia in this group, particularly with prolonged linezolid treatment [9,17–27]. Furthermore, an exposure-response relationship has been observed for linezolid-induced thrombocytopenia, with the prevalence of thrombocytopenia increasing proportionally to higher trough concentrations of linezolid, particularly when these levels exceed 7 to 10 mg/L [12,13,26,28–30]. Previous investigations have revealed additional risk factors for thrombocytopenia, including advanced age, low body weight, extended treatment duration, low platelet count at initiation, and hepatic dysfunction [20,31–37]. However, there have been no consistent findings addressing the key factors contributing to thrombocytopenia in linezolid patients. Furthermore, most known toxicity studies have not employed time-to-event analyses, which could provide insights regarding the impact of long-duration treatment on the emergence of thrombocytopenia.
As a result, identifying the risk factor and taking immediate action are critical for reducing the prevalence of linezolid-induced thrombocytopenia and its associated clinical and economic cost. This would allow for more effective and safer use of linezolid, ultimately prolonging its utility in the era of emerging antimicrobial resistance.
Moreover, time-to-event analyses are critical for investigating how treatment duration affects the occurrence of this adverse event. This study aimed to determine the prevalence of thrombocytopenia in patients with and without renal impairment and to identify the risk factors associated with linezolid-induced thrombocytopenia.
2. MATERIALS AND METHODS
This study followed the parameters indicated in the STROBE checklist (provided in Supplementary Table S1) [38]. We carried out a retrospective cohort analysis at University Medical Center Ho Chi Minh City, Viet Nam. Data were collected from patient electronic medical records, which included demographic information, type of infection, microbiological isolates, previous glycopeptide use, linezolid dosage, daily per kg dose of linezolid (mg/kg/12h), hemodialysis (yes/no), duration of linezolid therapy, and hematological (hemoglobin, platelet) and biochemical findings (total protein, albumin, serum creatinine, aspartate transferase, alanine transferase, total bilirubin), withdrawal linezolid (yes/no), platelet recovery (yes/no/missing data), the time from linezolid withdrawal to platelet recovery, co-medications. Patients were tracked longitudinally from the start of linezolid therapy until the end of treatment, including any outpatient visits.
We enrolled all patients who met the inclusion criteria, which included being aged 18 years or older who have received linezolid therapy for at least 7 days, including both inpatient and outpatient treatment. Patients were disqualified if they possessed any of the following: a diagnosis of disseminated intravascular coagulation or other bleeding complications; clear alternative causes for thrombocytopenia; abnormal baseline laboratory values, including an absolute neutrophil count<500 cells/microL, total bilirubin>5 times the upper limit of the normal, platelet count<75×103 cells/μL, or hemoglobin<6.8 g/dL for males and <6 g/dL for females; absence of baseline height/weight or laboratory data; and lack of a repeat platelet count after day 3; treatment in the intensive care unit at any point during admission; a variation in estimated glomerular filtration rate (eGFR) using the chronic kidney disease epidemiology collaboration (eGFR CKD-EPI) equation exceeding 50% after linezolid administration; or receipt of blood or other blood products.
The study size was estimated based on the difference in the prevalence of thrombocytopenia between patients with and without renal impairment, using the following equations:
Based on findings from previous research and our pilot study, we selected p1 and p2 as the prevalence of thrombocytopenia among patients with normal renal function (0.2) and those with renal impairment patients (0.4), respectively. The type 1 error and type 2 errors were set at α=0.05 and β=0.2, respectively, yielding a sample size of 164 cases [20,22,27]. To meet the sample size requirement, patients admitted to the hospital from January 2023 to December 2023 were screened to select eligible participants.
Hematological and biochemical parameters were measured at any time point following at least 4 days of linezolid treatment. This timeframe was established based on previously reported onset times, the suggested mechanism of linezolid-induced thrombocytopenia, and the physician’s experience with each patient. This approach aims to accurately reflect the impact of linezolid on the development of thrombocytopenia. Thrombocytopenia was defined as a 25% reduction from the baseline level [21,26,39,40]. Patients were categorized as having thrombocytopenia at the first occasion when their measured platelet count dropped below the predetermined threshold of a 25% reduction from the baseline level, and they were censored thereafter. To assess the effect of renal function on the development of thrombocytopenia, patients were categorized into two groups based on their eGFR (CKD-EPI), those with eGFR<60 mL/min per 1.73 m2 and those with eGFR≥60 mL/min per 1.73 m2. The eGFR threshold of 60 mL/min per 1.73 m2 is a frequent cut-off modified medication dose. The baseline laboratory values, specifically platelet counts, are those that are normally measured prior to starting linezolid therapy. This count can be acquired on the day treatment begins; if this measurement is unavailable, the value recorded within three days prior to the commencement of treatment is utilized. The onset time of thrombocytopenia was defined as the first day when the criteria for diagnosing thrombocytopenia were satisfied. A low baseline platelet count was defined as less than 150×103 cells/μL. Platelet recovery was defined as the restoration of the platelet count to the normal range or the baseline value from its nadir.
All analyses were performed using RStudio version 4.3.2. Descriptive statistics were obtained using Student’s t-test for continuous variables that followed a normal distribution, while the Mann-Whitney U test was applied for those that did not. The Shapiro-Wilk test was used to determine normality. Continuous variables with a normal distribution were given as mean and standard deviation, while non-normally distributed data were represented as the median and interquartile range. Categorical variables were analyzed using Chi-squared test or Fisher's exact test. The prevalence of thrombocytopenia between the two renal groups was analyzed using the Chi-squared test. A p-value of less than 0.05 indicated a statistically significant difference. Kaplan-Meier were used to depict the time to beginning of thrombocytopenia, which was then statistically analyzed using the log-rank test. Multivariable Cox regression analysis was then conducted to control for potential confounding variables affecting the association between renal impairment and thrombocytopenia risk. The final model was chosen using the Bayesian model averaging (BMA) method. Missing data were resolved via the multiple imputation method, specifically employing the MICE function in RStudio.
This study was conducted in compliance with legal requirements and the principles outlined in the Declaration of Helsinki. The study protocol was reviewed and approved by the Institute Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City, under the assigned code 853/HĐĐĐ-ĐHYD.
3. RESULTS
A total of 170 patients were participated in the study, with 60 (35.5%) experiencing thrombocytopenia. The baseline characteristics of the overall study population are summarized in Table 1. The median age was 69.5 years, and 61.2% of the participants were male. The median (interquartile range) weight, height, and body mass index were 57.0 (50.0, 65.8) kg, 160 (156, 165) cm, and 22.3 (19.5, 25.2) kg/m2, respectively.
The median (interquartile range) eGFR for the entire population were 74.0 (45.3, 96.3) mL/min per 1.73 m2. Patients with thrombocytopenia had a significantly lower eGFR (median [interquartile range]) of 63.0 (40.5, 88.2) mL/min per 1.73 m2, compared to 78.0 (55.2, 99.8) mL/min per 1.73 m2 in the non-thrombocytopenia group (p=0.022). The median (interquartile range) serum creatinine levels were also higher in the thrombocytopenia group at 0.98 (0.76, 1.52) mg/dL, compared to 0.89 (0.68, 1.26) mg/dL in the non-thrombocytopenia group (p=0.038). The thrombocytopenia group had a substantially longer hospital stay (median [nterquartile range]) at 19.8 (15.0, 29,2) days compared to 14.0 (10.0, 18.0) days in the non-thrombocytopenia group (p<0.001).
The most common reasons for linezolid use were skin and soft tissue infections (38.8%), community-acquired pneumonia (29.4%), and bone and joint infections (10.6%). Baseline laboratory values for total bilirubin, aspartate aminotransferase, alanine aminotransferase, albumin, and hemoglobin were not statistically different between the thrombocytopenia and non-thrombocytopenia groups. However, the baseline platelet count (median [interquartile range]) was higher in the thrombocytopenia group at 326 (243, 407)×103 cells/μL, compared to 264 (183, 330)×103 cells/μL in the non-thrombocytopenia group (p=0.001).
The characteristics of linezolid treatment in the study population are presented in Table 2. Approximately one-third (32.9%) of patients received linezolid-targeted treatment. Of these cases, 24.7% was caused by MRSA and methicillin-resistant coagulase-negative Staphylococci infections. The median (interquartile range) linezolid dose given was 10.5 (9.1, 12.0) mg/kg/day, with no significant difference between the thrombocytopenia and non-thrombocytopenia groups. In 24.7% of cases, linezolid treatment was followed by vancomycin administration. Importantly, the duration of linezolid treatment was significantly longer in the thrombocytopenia group compared to the non-thrombocytopenia group. The median (interquartile range) duration was 14.0 (10.0, 21.0) days in the thrombocytopenia group, compared to 10.0 (8.0, 14.0) days in the non-thrombocytopenia group (p<0.001).
IV, intravenous infusion administration; PO, oral administration; MRSA, methicillin-resistant Staphylococcus aureus; MSSA, methicillin-sensitive S. aureus; MS-CoNS, methicillin-sensitive coagulase-negative Staphylococci; MR-CoNS, methicillin-resistant coagulase-negative Staphylococci; NA, not applicable.
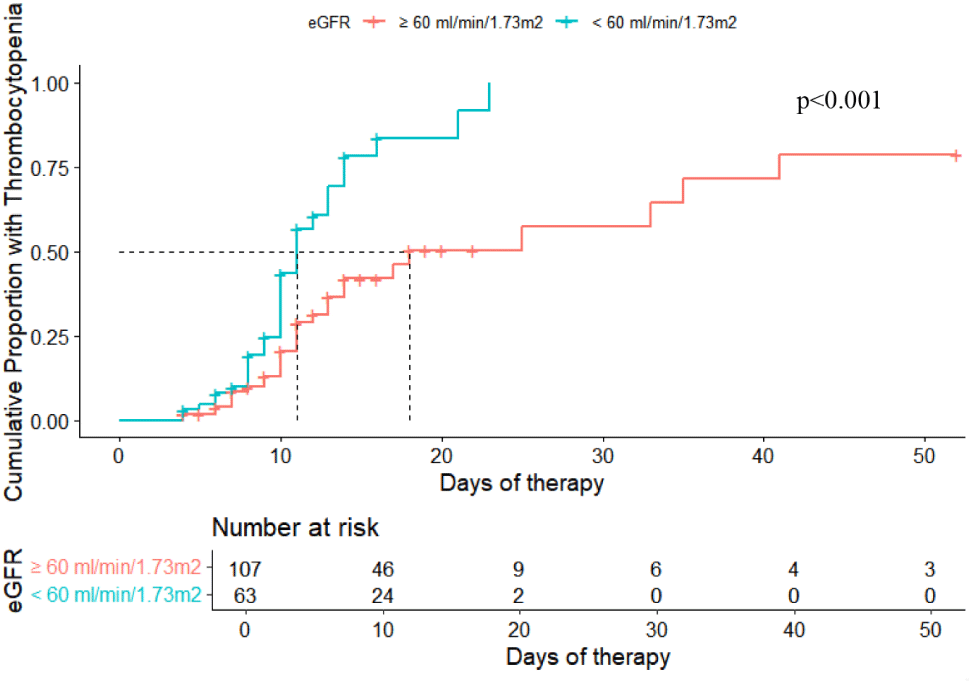
In this study, thrombocytopenia occurred in 60 (35.3%) of the 170 patient participated. The median time between the commencement linezolid therapy and the onset of thrombocytopenia was 14 days. Further analysis revealed that 23.3% (14 of 60) of patients experienced thrombocytopenia within the first 7 days, 61.7% (37 of 60) between 7–14 days, and 15% (9 of 60) after 14 days of linezolid treatment. Kaplan-Meier analysis was used to analyze the difference in the proportion of thrombocytopenia between groups based on eGFR (<60 mL/min per 1.73 m2 and ≥60 mL/min per 1.73 m2). The results demonstrated a significantly higher cumulative probability of thrombocytopenia events in the eGFR<60 mL/min per 1.73 m2 group (47.6%) compared to the eGFR≥60 mL/min per 1.73 m2 group (28.0%) (p<0.001). Additionally, the median time to thrombocytopenia was shorter in the eGFR<60 mL/min per 1.73 m2 group (11 days) compared to the eGFR≥60 mL/min per 1.73 m2 group (18 days). These results are displayed in Fig. 1. The predominant reason for linezolid removal was an underlying indication, such as completion of the intended treatment course, de-escalation of therapy, or therapeutic failure, which occured in both the non-thrombocytopenia (98.2%) and thrombocytopenia (65%) groups. A minority of individual patients with thrombocytopenia (25%) stopped using linezolid due to a combination of adverse medicaiton reactions and indications. Among patients who developed thrombocytopenia, 41.7% experienced platelet recovery, with a median time of 7.0 days, ranging from 3.0 to 13.0 days after linezolid discontinuation.
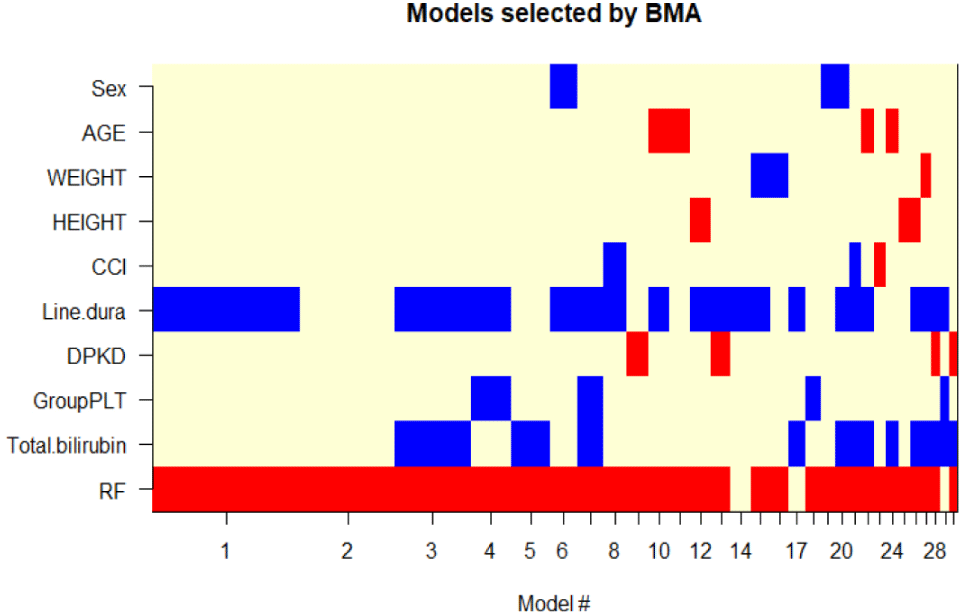
To identify factors potentially influencing the development of thrombocytopenia, a Cox proportional hazards model analysis was conducted. All relevant variables were included, such as sex, age, weight, height, Charlson comorbidity index, duration of linezolid treatment, linezolid dose (dose per kg per day), low baseline platelet count (defined as platelet count<150×103 cells/μL), high baseline total bilirubin (defined as total bilirubin≥20.5 umol/L), and renal impairment status (defined as eGFR<60 mL/min per 1.73 m2). These factors were chosen based on their documented effect on linezolid-induced thrombocytopenia in previous literature [9,10] their physiological relevance, and the unequal distribution between thrombocytopenia and non-thrombocytopenia groups, as presented in Tables 1 and 2. The BMA method was then utilized to determine the best model among these variables.
The results showed that the model including two variables, renal impairment status and length of linezolid therapy, was the best model for predicting thrombocytopenia, with a Bayesian Information Criterion of –8.93 and a posterior probability of 25.2% (presented in Fig. 2). This posterior probability indicates that there is a 25.2% chance that this model is accurately represents the link between the variables and thrombocytopenia, making it most plausible of the evaluated combinations of variables. Furthermore, the study found that renal impairment status had greater influence on the development of thrombocytopenia than linezolid treatment length. In the final model, the adjusted hazard ratio (aHR) for renal impairment was 2.287 (95% confidence interval [CI] 1.328–3.907, p=0.0028), whereas the aHR for the duration of linezolid therapy was 0.972 (95% CI 0.946–0.999, p=0.0396). Table 3 presents a detailed univariable and multivariable Cox proportional hazards model analysis of thrombocytopenia.
4. DISCUSSION
Linezolid has been recognized as an effective treatment for Gram-positive infections for nearly 25 years, thanks to its advantages such as convenient dosing, high oral bioavailability which allows for easy transitions from intravenous to oral administration, and a unique mechanism of action that reduces the likelihood of bacterial resistance. However, thrombocytopenia is a major safety risk in clinical settings. The processes underlying linezolid-induced thrombocytopenia are not well understood, making it critical to explore the risk factors associated with linezolid therapy. Our retrospective cohort study provides a high prevalence of thrombocytopenia (35.3%) among patients receiving linezolid, with a median onset occuring 14 days after treatment initiation. Furthermore, our results identify key risk factors for thrombocytopenia, including renal impairment status and extended duration of linezolid therapy. These findings are critical in guiding physicians in making proper therapeutic decisions for linezolid treatment.
The linezolid-induced thrombocytopenia prevalence reported in previous studies ranges from 13.9% to 60.5%, with the definition of thrombocytopenia variably as platelet count less than 100 to 150×103 cells/μL and/or a reduction of 20%–50% from the baseline platelet count [9,10]. In our study, the prevalence of thrombocytopenia was 35.3%, and it was considerably greater in a group of renal impairment patients than that of normal renal function group. These findings are consistent with the previously reported literature. However, the proportion of thrombocytopenia varied widely between trials. This can be attributed to a variety of factors, including the definition of thrombocytopenia, the inclusion criteria of study subjects, and the duration of follow-up after initiating linezolid therapy. Firstly, there is a difference in the definition of thrombocytopenia utilized across different studies. Most studies use criteria such as a decrease below 100–150×103 cells/μL if the baseline platelet count is within the normal range, or a decrease of at least 25%–30% from the baseline count when the baseline is less than 150×103cells/μL [20,28,29,34,39,41–43]. These criteria offer flexibility and the capacity to distinguish patients with normal platelet counts at baseline from those with abnormal counts. However, the threshold of a reduction below 100–150×103cells/μL may be too stringent for patients with baseline platelet counts within the normal range, potentially leading to delays in diagnosis and provision of supportive interventions, particularly in cases where patients have high baseline platelet levels. In contrast, using less strict criteria, such as a 25%–30% fall in platelet count from baseline, regardless of the original level, can aid in the identification of more cases with thrombocytopenia. These criteria are easily applicable in clinical practice and can prevent delays in diagnosis, ensuring timely recognition of severe thrombocytopenia and facilitating prompt treatment and supportive measures. Some researchers propose defining thrombocytopenia as a declining platelet count below 100–150×103 cells/μL regardless of platelet baseline level [17,19,44]. However, this definition may lead to an artificially high prevalence of thrombocytopenia because it initially includes patients with low platelets (<100–150×103 cells/μL for instance) at first and conclude them as having thrombocytopenia. Therefore, in this study, we used the criteria of 25% decrease in platelet count from a baseline value to identification of thrombocytopenia. This approach helps avoid overestimating the prevalence of thrombocytopenia by taking into consideration for patients' baseline platelet status and focusing on substantial platelet count declines. To ensure the validity of our findings, we employed rigorous inclusion criteria for study subjects. First, we enrolled only non-intensive care unit (ICU) patients in order to reduce the impact of acute infection phases on platelet values. This enables us to ascribe platelet counts changes to the effects of linezolid therapy rather than to random oscillations. We also required patients to have stable renal function, with a maximum 50% variation in eGFR values between starting and finishing linezolid treatment. This criterion allowed us to precisely assess the impact of renal function on the development of thrombocytopenia. Additionally, eliminating patients receiving blood or blood component therapy reduced bias in thrombocytopenia assessment. Implementing these criteria allowed us to create a more homogeneous patient sample, reduce the impact of confounding variables, and improve the overall validity of our findings.
Our study found that the beginning of thrombocytopenia among patients who developed this side effect occurred at a median of 14.0 days. Further investigation revealed a more specific timeframe for the onset of thrombocytopenia. Specifically, 23.3% of patients using linezolid had thrombocytopenia within the first 7 days of therapy, 61.7% between days 7 and 14, and 15.0% after 14 days. Interestingly, the median onset time was significantly shorter in patients with renal impairment, at 11 days, compared to 18 days for those with normal renal function. These findings are consistent with the previous research, which indicated that thrombocytopenia generally developed within 10 to 14 days after initiating linezolid therapy, and this incidence did not increase proportionally with longer treatment durations [9,26–28,45]. The timing of thrombocytopenia onset is likely associated with the underlying causes. For instance, immune-mediated thrombocytopenia tends to cause a rapid decline in platelet count, with onset ranging from a few hours to 1–2 weeks [14,15]. In contrast, if the decrease is due to inhibition of mitochondrial protein synthesis, suppression of precursor cell proliferation, or reduced precursor cell production, the reduction typically occurs more gradually over a period of 2 to 4 weeks [12,13,46]. Another proposed mechanism involves reduced antioxidation, increased lipid peroxidation, and free radicals, explored in patients treated for over 20 days [16]. These underlying mechanisms may be intensified by accumulated linezolid concentrations, which can be higher in renal impairment patients or those taking long-term linezolid treatment, as these factors encourage drug accumulation.
Research has consistently shown that the development of linezolid-induced thrombocytopenia is influenced by linezolid exposure, as indicated by two pharmacokinetic/pharmacodynamic indices, including Cmin and AUC24. Initial evidence suggested a relatively high Cminthreshold for thrombocytopenia, varying between 14.4 and 35.6 mg/L [41]. However, more recent studies have found significantly lower Cmin levels associated with the development of thrombocytopenia. Results from Matsumoto et al. indicated that a linezolid concentration below 8.2 mg/L could reduce the risk of developing thrombocytopenia-related adverse events [28]. Furthermore, Boak et al. developed a pharmacokinetic/toxicodynamic model showing that the synthesis of platelet precursor cells could be inhibited by 50% when the linezolid concentration exceeds 8.1 mg/L [12]. Additionally, Cattaneo et al. also suggested a threshold of >9 mg/L for Cmin, and >400 mg · h/L for AUC24 that are correlated with the development of thrombocytopenia [47,48]. Among these pharmacokinetic/pharmacodynamic parameters, Cmin is preferred for predicting linezolid-induced adverse reactions in clinical practice due to its availability and ease of application.
In addition to linezolid concentration, several risk factors associated with linezolid-induced thrombocytopenia have been discovered. Our study’s multiple Cox proportional hazard analyses reveal that renal impairment and the length of linezolid therapy are significant contributors to the development of thrombocytopenia in treated patients. Specifically, the analyses indicate that renal impairment (aHR 2.287; 95% CI 1.328–3.907) and the duration of linezolid therapy (aHR 0.972; 95% CI 0.946–0.999) are independently associated with thrombocytopenia. These findings align with earlier studies that also recognized these variables as risk factors for linezolid-induced myelosuppression [18,19,22,23,27,35,39,49]. The relationship between thrombocytopenia and the duration of linezolid therapy may be attributed to the primary mechanism of action, which involves the inhibition of mitochondrial protein synthesis. This effect is likely exacerbated as linezolid accumulates over time, causing increasing mitochondrial toxicity and a subsequent drop in platelet count. Additionally, factors that accelerate linezolid accumulation, such as renal impairment, hepatic impairment, or elderly, may further contribute to a more rapid decrease in platelet levels. According to linezolid labeling, the recommended treatment duration is 10–14 days for pneumonia, bacteremia, and skin and soft tissue infections, while it is 14–28 days for those caused by vancomycin-resistant Enterococcus faecium [5]. However, using linezolid for over 28 days, particularly in severe infections such as prosthetic joint infection, osteomyelitis, or meningitis, has not been evaluated in controlled clinical studies and raised safety concerns. This highlights the importance of close monitoring of platelet counts in high-risk patients throughout treatment. Proactive dose modification guided by therapeutic drug monitoring or switching to alternative antimicrobial agents may be essential to mitigate the risk of thrombocytopenia and other major adverse drug reactions.
Renal impairment is widely recognized as a significant risk factor for linezolid-induced thrombocytopenia [9,10,50]. However, the results from previous studies have been inconsistent in identifying renal dysfunction as an independent factor for thrombocytopenia. The approved label recommends that dose adjustments are not necessary for patients with impaired renal function. This conclusion is based on a pharmacokinetic study involving healthy volunteers who received a single dose of linezolid, which found no significant differences in linezolid clearance across varying levels of renal function [1,2,5]. The argument was that linezolid is removed by both renal and non-renal pathways, allowing them to compensate for one another when renal elimination is restricted by renal failure. Furthermore, any differences were minor and had no impact on the safety outcomes during the clinical trail’s brief follow-up period. However, many studies using real-world data have provided opposite evidence, indicating that renal function can significantly influence linezolid pharmacokinetic parameters. Specifically, impaired renal function decreases clearance values, leading to increased cumulative linezolid concentrations, which have been demonstrated to be linked to the development of thrombocytopenia. To be more specific, results from a meta-analysis indicated that the risk of thrombocytopenia was more than twice as high in patients with renal impairment, severe renal impairment, and those on hemodialysis compared to individuals with normal renal function, with odds ratios of 2.51, 2.38, and 3.34, respectively [10]. To ensure the safety of linezolid, optimum empirical dosage regiments for renal dysfunctional individuals must be established, as well as drug concentrations monitored during treatment.
We recognize that this study has a few limitations. First, we did not collect linezolid concentration data, which prevented us from establishing the relationship between linezolid exposure and thrombocytopenia. Second, the study is subject to limitations inherent to its retrospective design.
5. CONCLUSION
In conclusion, linezolid therapy is associated with a significant prevalence of thrombocytopenia. Both renal impairment and the duration of linezolid therapy have been identified as major risk factors for linezolid-induced thrombocytopenia. As a result, to preserve the efficacy and safety of linezolid therapy, these high-risk patients may require empirical dose modification and therapeutic drug monitoring throughout the treatment.
