1. INTRODUCTION
Gastric ulcers are one of the most common gastrointestinal disorder, affecting approximately 5%–10% of the global population, with incidence rates rising rapidly [1]. Gastric ulcers result from an imbalance between protective factors and destructive factors of the gastric mucosa, resulting in mucosal damage from gastric acid, which can extend to the submucosa or muscular layers of the stomach [2]. Several factors contribute to this damage, such as Helicobacter pylori infection, alcohol and tobacco use, non-steroidal anti-inflammatory drugs, and psychological stress [2,3]. These factors increase reactive oxygen species (ROS), leading to an oxidative stress, which either promotes more ROS production or reduces antioxidant defense mechanism in the stomach [3]. As a result, antioxidants are considered potential therapeutic agents in development of new drugs for treating gastric ulcers.
L. octovalvis (Jacq.) from the Onagraceae family has long been used in traditional medicine for its cooling properties and mild sweet taste. This medical herb is known for its ability to eliminate dampness, clear heat, reduce swelling, alleviate inflammation, and effectively support the treatment of diarrhea and dysentery. Additionally, according to folk medicine, L. octovalvis is also used to treat H.pylori -induced gastric ulcers. The plant is dried, lightly roasted, and then decocted in water, to be consumed daily to improve stomach symptoms. Alternatively, the fresh plant can be soaked in diluted saline solution for disinfection and cleaning, then crushed with filtered water and consumed twice daily [4]. Several studies have documented the gastroprotective effects of L. octovalvis. In 2005, Taiwanese researchers screened various herbs, including L. octovalvis, for their antibacterial activity against H. pylori [5]. Kanto and colleagues found that a hydroalcoholic extract from the leaves of L. octovalvis significantly prevented ulcers in rats with pylorus ligation and indomethacin-induced ulcers at doses of 150, 300, and 600 mg/kg. The extract reduced gastric mucosal lesions and acid content in a dose-dependent manner, indicating its potential as an effective anti-ulcer agent by reducing acidity and enhancing gastric mucosa protection [6]. Furthermore, the 80% methanol extract of L. octovalvis has been reported to possess immunomodulatory hepatoprotecive and cardioprotective properties [7]. In addition to its anti-ulcer properties, L. octovalvis also exhibits inhibitory activity against digestive enzymes, including α-amylase, α-glucosidase, lipase, and pepsin, as demonstrated by in vitro assays [8]. These pharmacological effects are attributed to the presence of flavonoids, phenolic compounds, polysaccharides, and amino acids in the plant [4,6–8].
Currently, no studies have investigated the gastroprotective effects of L. octovalvis collected in Vietnam. This study aimed to examine both the antioxidant effects in vitro and gastroprotective effects in vivo of two types of extracts: a 96% ethanol extract, which may provide insights into its pharmacological effects and potential combination with other medicinal herbs in the treatment of gastric ulcers, and a water extract, which could be developed into a tea-based product for commercialization and to enhance the value of this medicinal plant.
2. MATERIALS AND METHODS
The aerial parts of L. octovalvis were collected from the wild populations in Vinh Long Province, Vietnam, between August and October 2023 and were identified by MSc. Ngo Thi Minh Huyen from the Department of Medicinal Material Resources - Research Center of Ginseng and Medicinal Materials, identification number TB-102023RM. After harvesting, the raw materials were washed with water, drained, then preprocessed by cutting into small pieces and naturally dried. The dried samples were then ground into powder (sieved through a 0.2 mm mesh). The processed material met in-house standards for moisture and total ash content.
Chemicals and reagents: 2,2-diphenyl-1-picrylhydrazyl (DPPH) was purchased from Aldrich-Sigma (St. Louis, MO, USA). Methanol was obtained from Merck (Darmstadt, Germany), ethanol from OPC (Ho Chi Minh City, Vietnam), formalin and HCl from Xilong (Guangdong, China), and omeprazole (Losec Mups 20 mg) from AstraZeneca (Sodertalje, Sweden).
The powdered materials were exhaustedly extracted with 96% ethanol or decoction with distilled water at a 1:15 ratio (powdered materials/solvents) (w/v), then concentrated using a water bath to obtain 96% ethanol extract and water extract with moisture content suitable for concentrated extracts (<20%). The in vivo test doses of the extracts were calculated based on the extract’s dry weight.
Six-week-old ICR male micewere obtained from the Biotechnology Center of Ho Chi Minh City. The animals were housed in the Animal Room of the Unit of Traditional Medicine and Pharmacy, Faculty of Traditional Medicine, University of Medicine and Pharmacy at Ho Chi Minh City. The mice were housed in standard rearing cages, each measuring 15 cm×20 cm×30 cm, with 10 mice per cage. The cages were fitted with wire mesh flooring to prevent fecal ingestion, and cleaning was conducted daily. During the experiment, the mice were kept in an air-conditioned facility with a 12-hour light/dark cycle at a temperature of 28±2℃ and relative humidity not to exceed 70%. They were provided with tap water and standard laboratory food ad libitum. The mice underwent a minimum 7 days acclimation period before the experiment. All the mice will then included in the study. Animals were excluded under the following conditions: (1) if they have been used in previous experiments and/or (2) if they are unhealthy or exhibit abnormal signs before the experiment. However, confounding factors such as the order of treatments and measurements or the location of animals/cages were not controlled.
In this study, the euthanasia method using CO2 dry ice was employed to minimize pain for the animals.
All the procedures related to animal in this study were conducted under the approval of the Animal Research Ethics Committee of the University of Medicine and Pharmacy at Ho Chi Minh City (No. 304/GCN-HĐĐĐNCTĐV on February 2, 2024) and following the ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines (see Supplementary 1) [9].
The free radical scavenging ability of the extracts was carried out according to the method of Mensor et al. [10]. The antioxidant activity (AA) of the sample was evaluated based on its ability to eliminate free radicals by reducing the color of DPPH. The assay was briefly performed as follows: Prepare a 0.2 mM solution of DPPH in methanol. Weigh 0.025 g of L. octovalvis extracts and dissolve in 5 mL of methanol, then dilute to create a series of concentrations: 10, 25, 50, 75, 100, and 125 µg/mL using methanol. Take 100 µL of each sample and react with 0.2 mM DPPH solution in methanol, incubate in the dark at room temperature for 30 minutes, and measure absorbance using a spectrometer at 540 nm. Ascorbic acid was used as positive control: weigh 0.016 g of ascorbic acid, dissolve in 100% methanol, and dilute to create a series of concentrations: 1, 2, 4, 8, and 16 µg/mL. The AA is calculated as follows:
where: Acontrol is the absorbance of the control sample and Asample is the absorbance of the test sample.
The experiment was conducted three times independently at each concentration and results are presented as averages. Using the AA values, a linear regression equation relating the sample concentrations and AA was constructed to determine the IC50 value.
Acute toxicity tests of the extracts were conducted using the model of Litchfield-Wilcoxon and following the OECD guidelines [11]. Briefly, six-week-old ICR male mice were divided into groups of 10. Each group received different doses of plant extracts, from the highest non-lethal dose to the lowest dose causing 100% mortality. The volume for oral or injection administration was set at 10 mL/kg of the mice’s body weight. The extracts were administered orally using a blunt ended, slightly curved needle inserted into the stomach. Mice were fasted 12 hours prior but had access to water. Mortality was monitored for the first 72 hours, and general conditions such as eating, neurological activity, movement, and excretion were observed for 7 consecutive days. In case of death, a necropsy was performed to assess gross organ damage, with microscopic examination if necessary. LD50 values of the extracts were calculated using the Litchfield-Wilcoxon method.
The daily dosage for fresh L. octovalvis in humans, as recorded in ethnopharmacological texts, is 20–40 g. Assuming an average body weight of 50 kg for humans, the daily dosage of fresh herbs is approximately 0.4–0.8 g/kg. The corresponding dose for mice was calculated using the formula [12]:
Thus, the daily dosage of fresh herbs for mice was approximately 5–10 g/kg. The dosage of the extract using in the next experiment was calculated using the following formula:
The resulting dosages are presented in Table 1. All doses met the criteria of being less than 1/10 of the Dmax, ensuring their suitability for subsequent experiments [12].
The HCl/Ethanol-induced acute gastric ulcers model was conducted using the method of Paguigan et al. [13]. Briefly, the mice were randomly divided into groups of 10 and fasted for 12 hours before administration. For 7 consecutive days, they received either distilled water or treatments at 10 mL/kg as follows:
-
- Physiological group (normal group): distilled water
-
- Pathological group (control group): distilled water
-
- OM group: omeprazole 20 mg/kg
-
- EL1 group: 96% ethanol extract of L. octovalvis at dose 0.7 g/kg
-
- EL2 group: 96% ethanol extract of L. octovalvis at dose 1.4 g/kg
-
- WL1 group: water extract of L. octovalvis at dose 0.5 g/kg
-
- WL2 group: water extract of L. octovalvis at dose 1.0 g/kg
On the seventh day, one hour after the final dose, all mice except the normal group were given 10 mL/kg 0.3 M HCl/60%EtOH orally to induce an acute gastric ulcer model. Two hours later, the mice were anesthetized with carbon dioxide, and gastric tissue was collected for anatomical and histopathological evaluation. For each mouse, drug administration, anesthesia, and stomach collection for macroscopic evaluation were performed by an investigator who was aware of the treatment group allocation. The samples will then be sent to the Department of Pathology at Cho Ray Hospital, Ho Chi Minh City, where a second investigator, blinded to the treatment group allocation, would prepare the samples and conduct the histological evaluation.
The removed stomach was rinsed in ice-cold 0.9% w/v NaCl solution (saline). The gastric ulcer index (GUI) was assessed by analyzing images taken with an iPhone 13 through a stereomicroscope. The stomach were evaluated and scored for pathology using the following scale: 0=no damage, 1=blood in the lumen, 2=pin-point erosions, 3=1–5 small erosions, 4=more than 5 small erosions, 5=1–3 large erosions (>2 mm), and 6=more than 3 large erosions [14]. The ulcer inhibition rate (UIR), a key measure of gastric mucosal damage, was calculated as follows [15]:
Histological analyses were conducted at the Department of Pathology, Cho Ray Hospital, Ho Chi Minh City. Five stomach samples per group were randomly selected for the analysis. The excised stomach was fixed in 10% formalin for at least 48 hours, dehydrated in graded alcohol, embedded in paraffin, and sectioned into 4 μm slices. Hematoxylin and eosin (H&E) staining was used to observe pathological changes in the gastric mucosa, focusing on congestion and cell mixtures, under an optical microscope at a HE ×200 magnification.
All the data were presented as mean±SEM. Data was analyzed using Sigmastat 3.5 software. The normal distribution of the data was assessed using the Shapiro-Wilk test, and homogeneity of variance was checked using Levene test. Weight variables were tested using one-way Analysis of variance (ANOVA) (or Kruskal-Wallis ANOVA on Ranks in cases of non-normal distribution or unequal of variance), followed by post hoc analysis with the Student-Newman-Keuls test. GUI index between groups were tested using one-way ANOVA (or Kruskal-Wallis ANOVA on Ranks in cases of non-normal distribution or heterogeneity of variance), followed by post hoc analysis with the Student-Newman-Keuls test. GUI index between two groups were tested using the t-test (or Mann-Whitney Rank Sum Test in cases of non-normal distribution or unequal of variance). Results were considered statistically significant at a 95% confidence level when p<0.05 compared to the respective controls.
3. RESULTS
The results of the acute oral toxicity test in mice (n=10) determined the maximum safe dose administered via gavage. The Dmax for the 96% ethanol extract and the water extract of L. octovalvis were 20.8 g/kg and 17.15 g/kg, respectively.
Based on the clinical human doses, the human-to-mice conversion factor, and the safe dose determined from the acute toxicity test, the experimental dose for pharmacological tests in mice was calculated. This dose (equivalent to 5 g and 10 g of dried medicinal material) was adjusted for extraction yield and moisture content as shown in Table 1.
Thus, the doses administered for the 96% ethanol extract of L. octovalvis (EL) in the pharmacological experiments were 0.7 g/kg (EL1), 1.4 g/kg (EL2); Water extract were 0.5 g/kg (WL1) and 1 g/kg (WL2), respectively.
Based on the results from the DPPH free radical scavenging assay results, linear regression equations between the concentration of the test samples to the AA of L. octovalvis extracts and ascorbic acid were constructed, as shown in Fig. 1. From these results, the IC50 values were determined to be 25.08 µg/mL for the 96% ethanol extract, 31.04 µg/mL for the water extract, and 6.6 µg/mL for ascorbic acid.
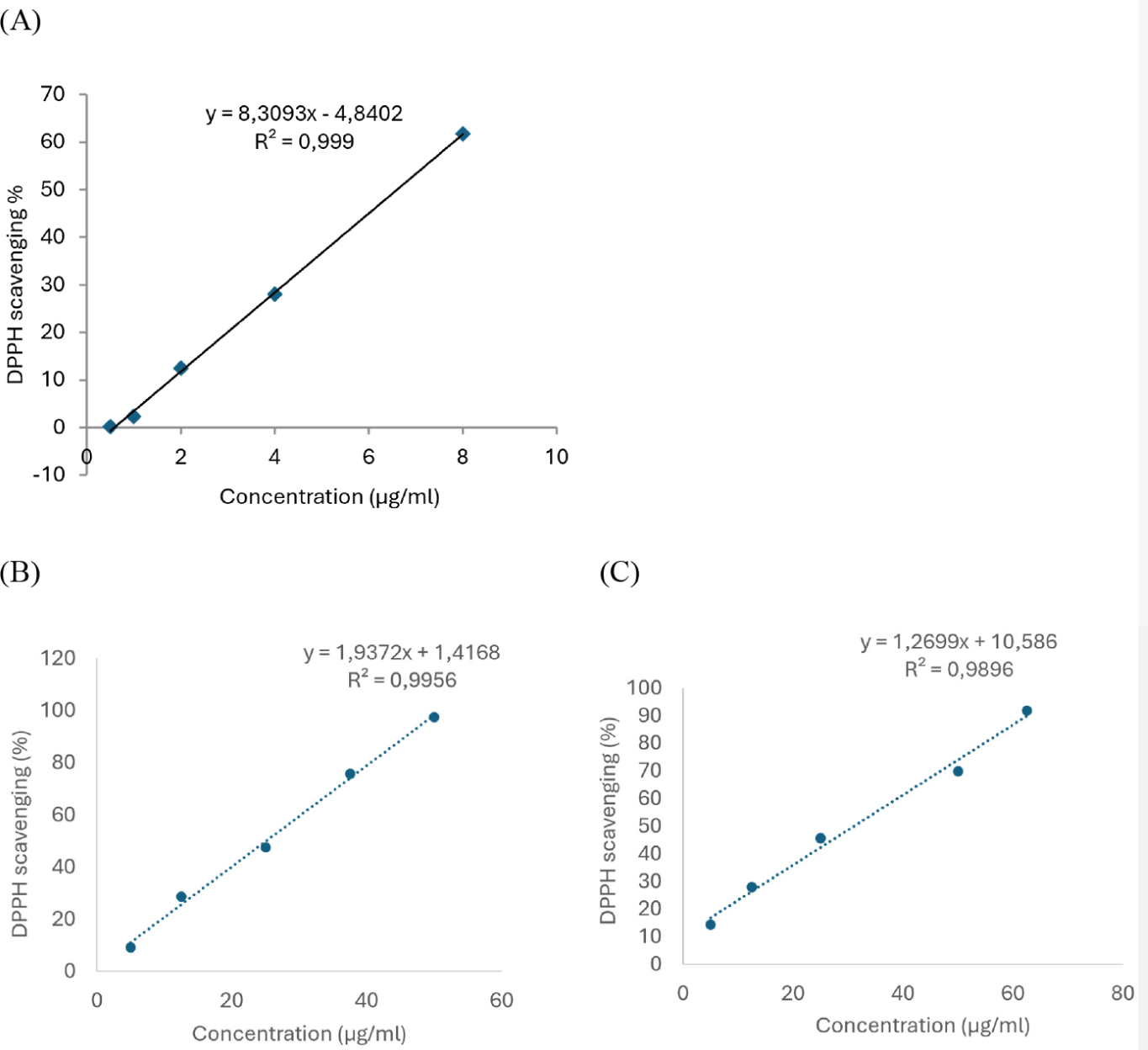
Both extract samples had IC50 values higher than ascorbic acid. However, the 96% ethanol extract had a lower IC50 compared to the water extract, indicating that the 96% ethanol extract has better AA than the water extract.
Body weight values in the groups on Day 1 followed a normal distribution (Shapiro-Wilk test, p=0.062) and had homogeneous variance (Levene test, p=0.790). There were no significant differences in body weight among the groups using L. octovalvis extracts and normal group (Kruskal-Wallis ANOVA test, p=0.867).
Body weight values in the groups on Day 7 did not follow a normal distribution (Shapiro-Wilk test, p<0.05) but had homogeneous variance (Levene test, p=0.802). There were no statistically significant differences in body weight among the groups using L. octovalvis extracts and normal group (Kruskal-Wallis ANOVA on Ranks test, p=0.898).
Thus, it is suggested that the L. octovalvis extracts did not affect the mice’s weight with prolonged use.
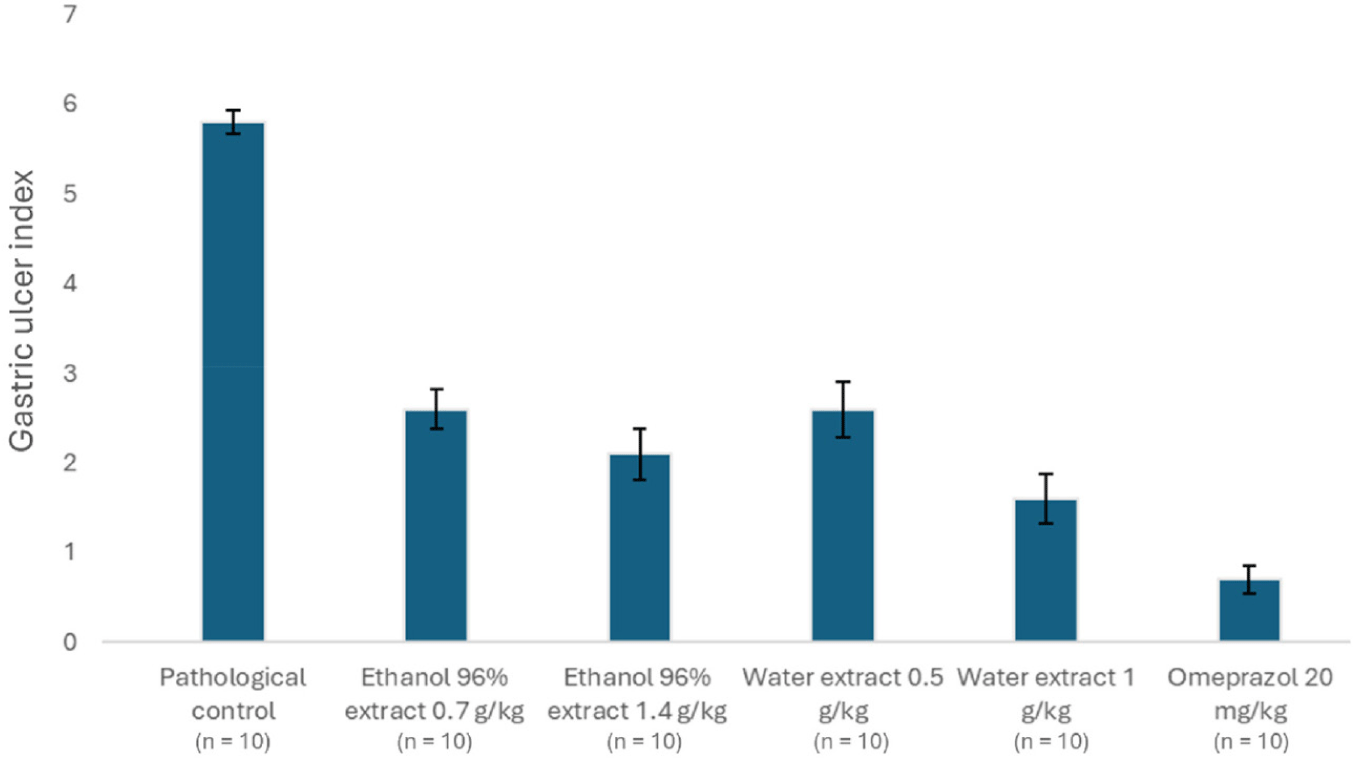
The gross images of the stomach are presented in Fig. 2. The GUI values in the pathological and physiological control groups did not follow a normal distribution (Shapiro-Wilk test, p<0.001) but had homogeneous variance (Levene test, p=0.151). As shown in Table 3 and Fig. 3, The GUI of the control group were significantly higher than those of the normal group (Mann-Whitney Rank Sum Test, p<0.001).
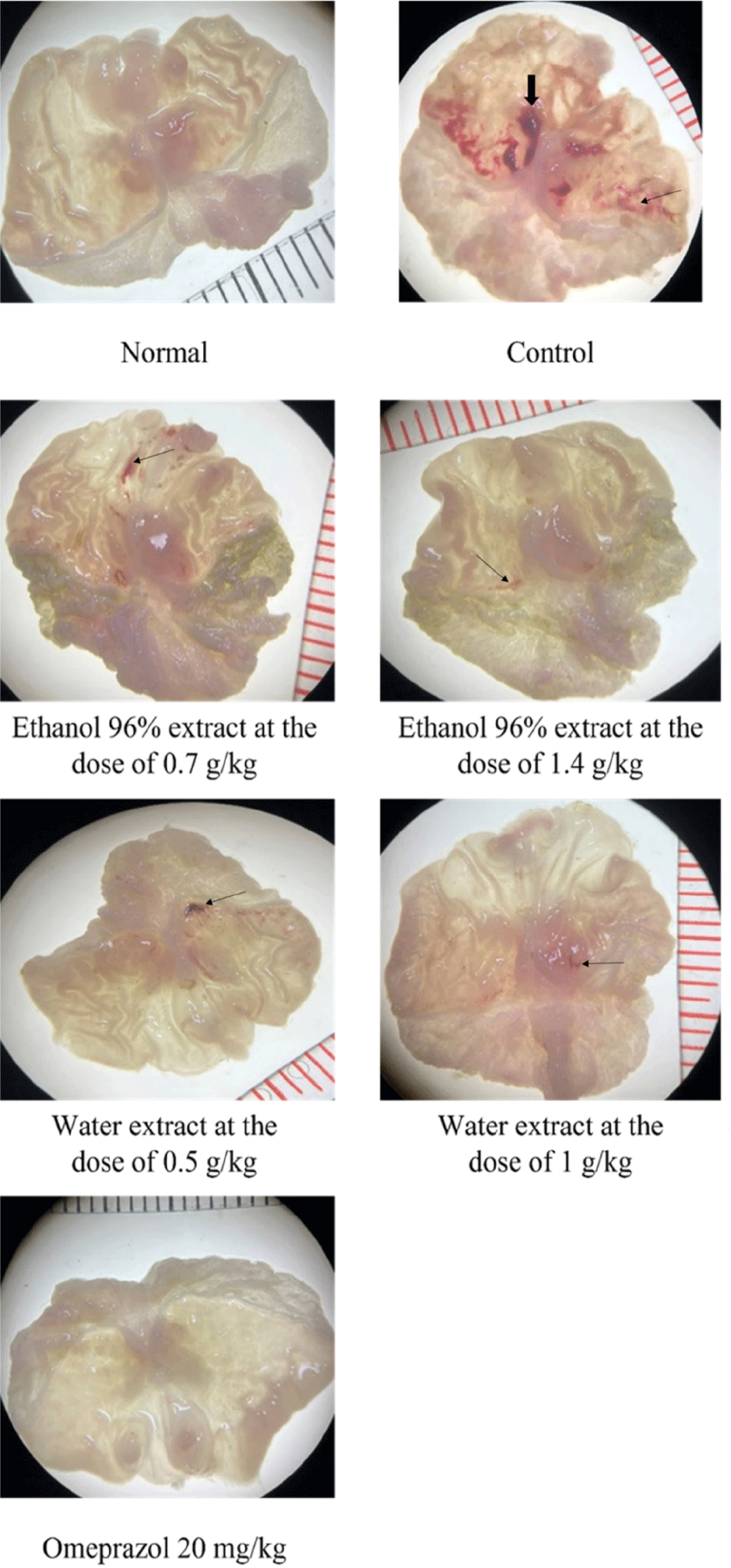
The GUI values compared to the pathological control group (Shapiro-Wilk test, p<0.05) but had homogeneous variance (Levene test, p=0.148). The differences between the groups were statistically significant (Kruskal-Wallis ANOVA on Ranks test, p<0.001). The treatment groups had significantly lower GUI values compared to the pathological control group (Student-Newman-Keuls test, p<0.05). Meanwhile, the group treated with omeprazole showed a significantly greater reduction in ulcer severity compared to the groups treated with the herbal extract (Student-Newman-Keuls test, p<0.05), indicating that the experimental model was suitable for investigating the gastric protective effects of the plant extracts (Table 3).
The ethanol extract of L. octovalvis at a dose of 1.4 g/kg reduced the GUI by 63.33%, which was higher than the reduction observed with the 0.7 g/kg dose (55%). However, the difference in GUI values between the two groups was not statistically significant (normal distribution with Shapiro-Wilk test, p=0.105; homogeneous variance with Levene test, p=0.591; and t-test, p=0.175). The aqueous extract of L. octovalvis at a dose of 1 kg reduced the ulcer index (72.33%) significantly greater than the reduction observed with the 0.5 kg dose (55.33%). Moreover, the difference in GUI values between the two groups was statistically significant (non-normal distribution with Shapiro-Wilk test, p<0.05; homogeneous variance with Levene test, p=0.523; and Mann-Whitney Rank Sum Test, p=0.03), indicating a dose-dependent effect. However, there was no significant difference between the 1.4 g/kg 96% ethanol extract and the 1.0 g/kg water extract (non-normal distribution with Shapiro-Wilk test, p<0.05; homogeneous variance with Levene test, p=0.749; and Mann-Whitney Rank Sum Test, p=0.209).
The histological images of the mouse gastric tissues are shown in Fig. 4. Table 4 presents the microscopic evaluation criteria for gastric tissue. The normal control group exhibited 100% normal gastric tissue, without ulcers or mucosal congestion. All stomach samples in the control group, which received ethanol orally, exhibited chronic active gastric ulcers, with 100% showing mucosal congestion and a high percentage of inflammatory cell infiltration in the mucosa (100%).
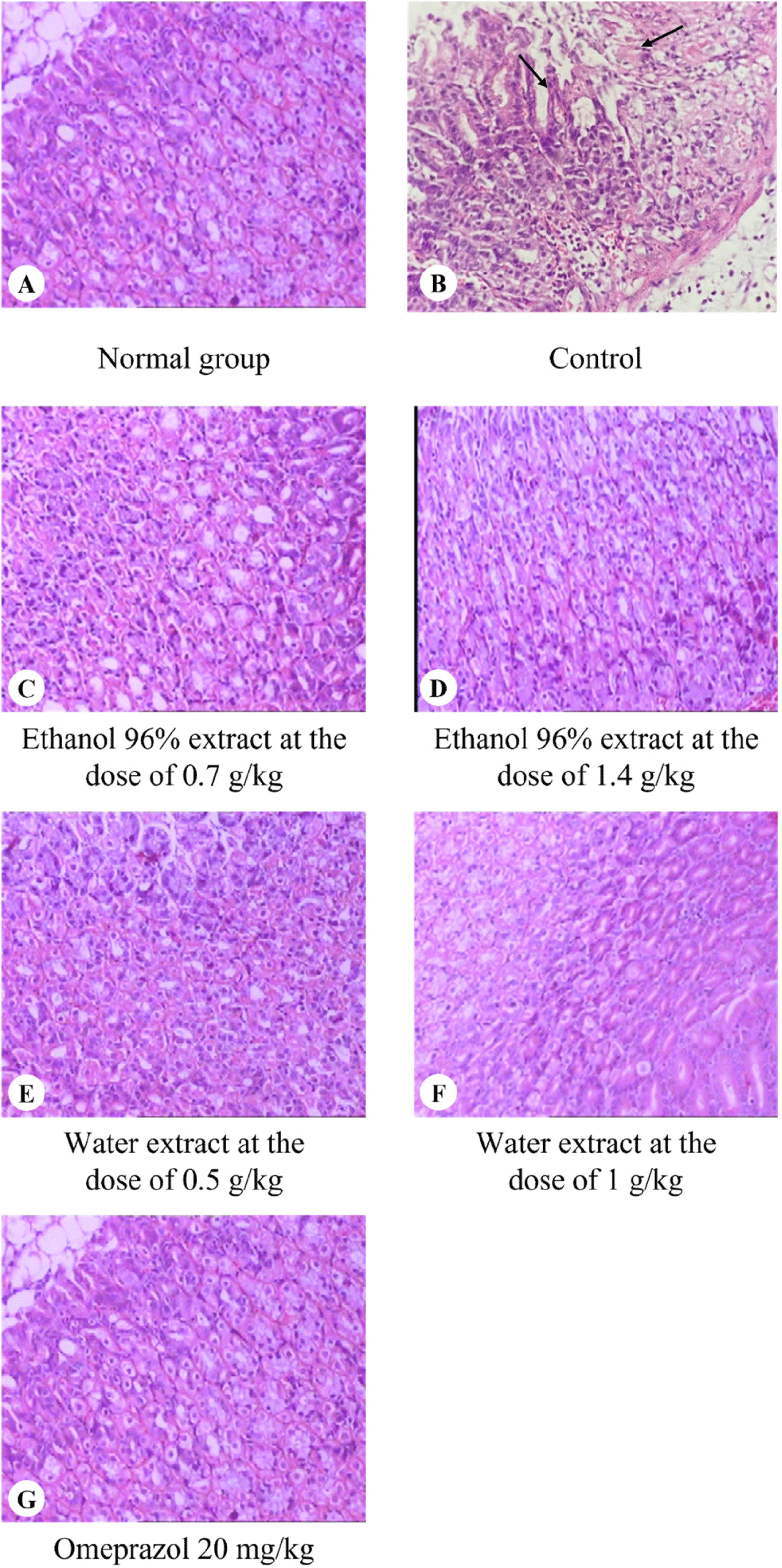
In the treatment groups with 96% ethanol extract of L. octovalvis at doses of 0.7 g/kg and 1.4 g/kg, there was a significant reduction in histopathological changes of gastric ulcers compared to the control group. At the dose of 1.4 g/kg, there were no cases of chronic active or moderate gastritis, or mucosal erosion. Meanwhile, these pathological features were still present in the gastric tissue of mice treated with 96% ethanol extract of L. octovalvis at a dose of 0.7 g/kg. This suggested that the ethanol extract at the dose of 1.4 g/kg provided greater gastroprotective effects when compared to 0.7 g/kg dose.
In the treatment groups with water extract of L. octovalvis at doses of 0.5 and 1.0 g/kg, there was also a significant reduction in histopathological changes of gastric ulcers compared to the control group. At the dose of 0.5 g/kg, there were no cases of chronic active or moderate gastritis, or mucosal erosion. At the dose of 1.0 g/kg, there was only mild chronic gastritis in 80% of the cases, indicating that the water extract of L. octovalvis at the dose of 1.0 g/kg exhibited better gastroprotective effects when compared to this extract at the dose of 0.5 g/kg.
4. DISCUSSION
Previous research has demonstrated that ethanol damages vascular endothelial cells in the gastric mucosa, leading to microcirculatory disturbances and hypoxia. This, in turn, disrupts the mitochondrial energy metabolism, resulting in in gastric mucous cell death. Consequently, antioxidants may serve as potential gastric protective agents, and certain antioxidants, such as rebamipide have been utilized in the treatment for gastric ulcers [3]. The in vitro DPPH assay results clearly demonstrated the AA of the 96% ethanol extract and water extract of L. octovalvis, with IC50 values of 25.08 and 31.04 µg/mL, respectively. Previous studies have also confirmed the antioxidant potential of L. octovalvis. Specifically, extracts from various parts of the plant exhibited free radical scavenging activity in the DPPH assay, with the 80% methanol extract from the leaves showing the strongest antioxidant effect [16]. These findings suggest that L. octovalvis possesses potential antioxidant properties, which may be beneficial in the treatment of peptic ulcers.
After ingestion, ethanol is rapidly absorbed through the gastric mucosa, where it dissolves the protective mucus, leading to protein degradation, increased HCl and pepsin production, and gastric mucosal damage. Additionally, ethanol stimulates acid secretion and reduces blood flow, causing microvascular damage through endothelial cell disruption and increased vascular permeability, along with enhanced xanthine oxidase activity [17]. Therapy for gastroduodenal ulcers aims to reduce acid secretion and/or enhance mucosal protection. Evaluating the damage caused by a 60% ethanol-induced gastric ulcer model, it was found that the 96% ethanol extract of L. octovalvis at doses of 0.7 g/kg and 1.4 g/kg, and the water extract of L. octovalvis at doses of 0.5 g/kg and 1.0 g/kg, both exhibited gastric mucosal protective effects through antioxidant properties. Notably, the water extract of L. octovalvis showed dose-dependent gastroprotective effects.
Seven days of oral prophylactic administration showed that L. octovalvis extracts effectively inhibited gastric ulcer formation, comparable to the positive control drug omeprazole. This study is the first to evaluate the anti-ulcer potential of L. octovalvis in a mouse model of HCl/ethanol-induced gastric ulcers. Previously, the anti-ulcer potential of hydroalcoholic extracts of L. octovalvis was evaluated in mice using pylorus ligation and indomethacin-induced gastric ulcer models [6]. These findings reinforce the anti-ulcer properties of L. octovalvis, laying the groundwork for future clinical investigations into its therapeutic efficacyin gastric ulcer treatment.
According to traditional medicine theory, the symptoms of gastric ulcers correspond to those described for the condition called Epigastric pain syndrome. One cause of Epigastric pain syndrome is the accumulation of dampness and heat in the spleen and stomach [18]. L. octovalvis typically grows in areas with mud or waterlogged fields (which means regions with a lot of dampness), but the plant itself is relatively dry and astringent, giving it the ability to eliminate dampness [4]. Its bland taste has diuretic effects, its cooling nature can clear heat, facilitates the medicine’s entry into the spleen meridian [19]. These characteristics suggest that L. octovalvis can clear heat and eliminate dampness in the spleen and stomach through urination, thereby improving the symptoms of gastric ulcers.
Pharmacological studies have shown that the flavonoid components of L. octovalvis contain several compounds such as vitexin, isovitexin, orientin, isoorientin, luteolin, and quercetin [20]. The results demonstrating improvement in GUI in this study suggest that L. octovalvis extract significantly reduces gastric acidity. Luteolin and quercetin are known to inhibit H+/K+-ATPase in parietal cells and reduce acid formation [21]. This suggests that L. octovalvis extracts can inhibit H+/K+-ATPase, reduce gastric acidity and serve as an effective anti-ulcer agent. Additionally, the extracts reduce the lesion surface area on the gastric lining induced by ethanol/HCl. The protective effect of flavonoid compounds on the stomach lining against damage by ethanol/HCl is related to their modulation of endogenous platelet-activating factor, enhancement of mucus production, and antihistaminic properties which reduces histamine levels and the ethanol-induced increase in mast cells. Furthermore, the gastroprotective effects of flavonoids are closely linked to their potent antioxidant properties. Specifically, studies have shown that quercetin, through mechanisms such as inhibition of lipid peroxidation, enhancement of mucosal non-protein SH compounds in glutathione peroxidase, and superoxide dismutase activities, as well as reduction of protein carbonyl compounds, significantly reduces the severity of ethanol-induced ulcers [22].
Based on these results, we hypothesize that the 96% ethanol and water extracts of L. octovalvis could be effective anti-gastric ulcer medications due to their ability to decrease acidity and strengthen the protective mechanisms of the gastric mucosa, similar to omeprazole. This effect could be attributed to the presence of flavonoids in the extracts.
5. CONCLUSION
The 96% ethanol extract (doses of 0.7 and 1.4 g/kg) and the water extract (doses of 0.5 and 1.0 g/kg) from L. octovalvis (the doses of extracts are equivalent to 5 g and 10 g of dried herb) demonstrated gastroprotective effects against HCl/ethanol-induced gastric damage by reducing GUI in both macroscopic and histological evaluations. The extracts also reduced and prevented ulcer formation and inflammation in an acute HCl/ethanol-induced gastric ulcers model, with the most notable effects seen in the water extract of L. octovalvis the dose of 1.0 g/kg.