1. INTRODUCTION
In-vitro maturation (IVM) involves collecting immature cumulus-oocyte complexes (COCs) at the germinal vesicle (GV) stage from antral follicles in ovaries that are either unstimulated or minimally stimulated [1]. These COCs are then cultured in vitro until they reach the metaphase II (MII) stage. From this point onwards, IVM oocytes and any resulting embryos are handled similarly to those produced through conventional ovarian stimulation.
Standard IVM procedures involve culturing COCs in a tissue culture-like medium enriched with a protein source such as albumin or serum, follicle-stimulating hormone (FSH), and potentially other additives like human chorionic gonadotropin (hCG), epidermal growth factor (EGF), estradiol, and/or cysteamine, under atmospheric oxygen for 1 to 2 days. Unfortunately, clinical progress using IVM has been slow because these culture conditions have largely remained unchanged since Edwards first described them over 50 years ago [1]. Many clinics still use the simple standard IVM method today, resulting in modest pregnancy outcomes [2]. This is partly because this type of IVM is non-physiological. Human oocytes from follicles larger than approximately 6 mm mature spontaneously when artificially removed from the ovary and placed in vitro, and therefore mature without the appropriate maternal signals that normally induce oocyte maturation in vivo [3]. For example, oocytes matured spontaneously in vitro are not exposed to the full ovulatory cascade of EGF-like peptides that induce oocyte maturation in vivo [3,4]. Therefore, there has been the need for a more sophisticated IVM system based on the latest understanding of in vivo oocyte maturation physiology [5,6].
Significant advances in our understanding of fundamental aspects of oocyte biology and ovulation from animal studies have led to novel approaches to IVM [7]. One notable development in IVM technology is the use of biphasic oocyte maturation approaches, such as IVM with a pre-maturation step, exemplified by capacitation IVM (CAPA-IVM) [4,8,9]. CAPA-IVM involves using the C-type natriuretic peptide (CNP) molecule to inhibit the oocyte’s type-3 phosphodiesterase for the pre-IVM stage, which is the first phase of biphasic IVM, maintaining oocyte meiotic arrest at the GV stage. The principle of pre-IVM allows is to synchronize oocyte nuclear and cytoplasmic maturation, thereby capacitating the oocyte for embryonic development. This review explores the principles, indications, methods, effectiveness, safety, and future use of biphasic IVM.
2. PRINCIPLES OF BIPHASIC IN-VITRO MATURATION
Biphasic IVM protocols consist of two in vitro steps: 1) a pre-IVM step (typically about 24 hours) intended to enhance GV oocyte development, and 2) an IVM step (typically about 30 hours for human oocytes) where the oocyte meiotically matures from GV to MII. Thereafter MII oocytes are treated as per conventional in-vitro fertilization (IVF).
Immature COCs are collected from any sized antral follicle from unstimulated women or women who have received 2 or 3 days of FSH-priming at any stage of the cycle (but typically in the early follicular phase). Triggering with hCG or a gonadotropin-releasing hormone agonist is incompatible with biphasic IVM.
In the pre-IVM phase, the oocyte is deliberately arrested at the GV stage in vitro using meiotic inhibitors. There is a substantial body of literature that reports the use of various regulators in animal pre-IVM systems to inhibit oocyte maturation in vitro, and these most commonly target cyclic guanosine monophosphate (cGMP) and/or cyclic adenosine monophosphate (cAMP), which are the central regulators of oocyte meiosis [10]. CNP acts by increasing COC cGMP and, because CNP is the natural meiotic inhibitor in the follicle, it has emerged as the preferred meiotic inhibitor used in human pre-IVM culture systems [4]. During the pre-IVM phase, the intact COC structure is preserved enabling continued cumulus cell (CC) support of oocyte development via gap junctions and provision of CC regulators in the medium. In the subsequent IVM phase, meiotic resumption is induced by EGF-like peptides (such as amphiregulin) and FSH. It is hypothesized that this is a more physiological form of oocyte maturation because it is the EGF-like peptides in particular that are the natural inducers of oocyte maturation within the follicle in vivo. Therefore, this mode of inducing oocyte maturation in CAPA-IVM differs from traditional IVM, where hCG is typically added to the IVM medium. This biphasic IVM system is intended to mimic some aspects of oocyte maturation in vitro that occur naturally in vivo better than standard spontaneous IVM culture systems. This is supported by extensive data showing improved embryo development and pregnancy outcomes using biphasic IVM in animal studies and, more recently, in clinical studies (as recently reviewed [7]).
3. INDICATIONS
Biphasic IVM is particularly beneficial for specific patient groups and clinical scenarios. These include polycystic ovary syndrome (PCOS), women with a high antral follicle count (AFC), normo-responders, fertility preservation, and women with gonadotropin-resistant ovary syndrome (ROS).
4. BIPHASIC IN-VITRO MATURATION METHODS
The clinical protocol for pre-IVM is shown Fig. 1.
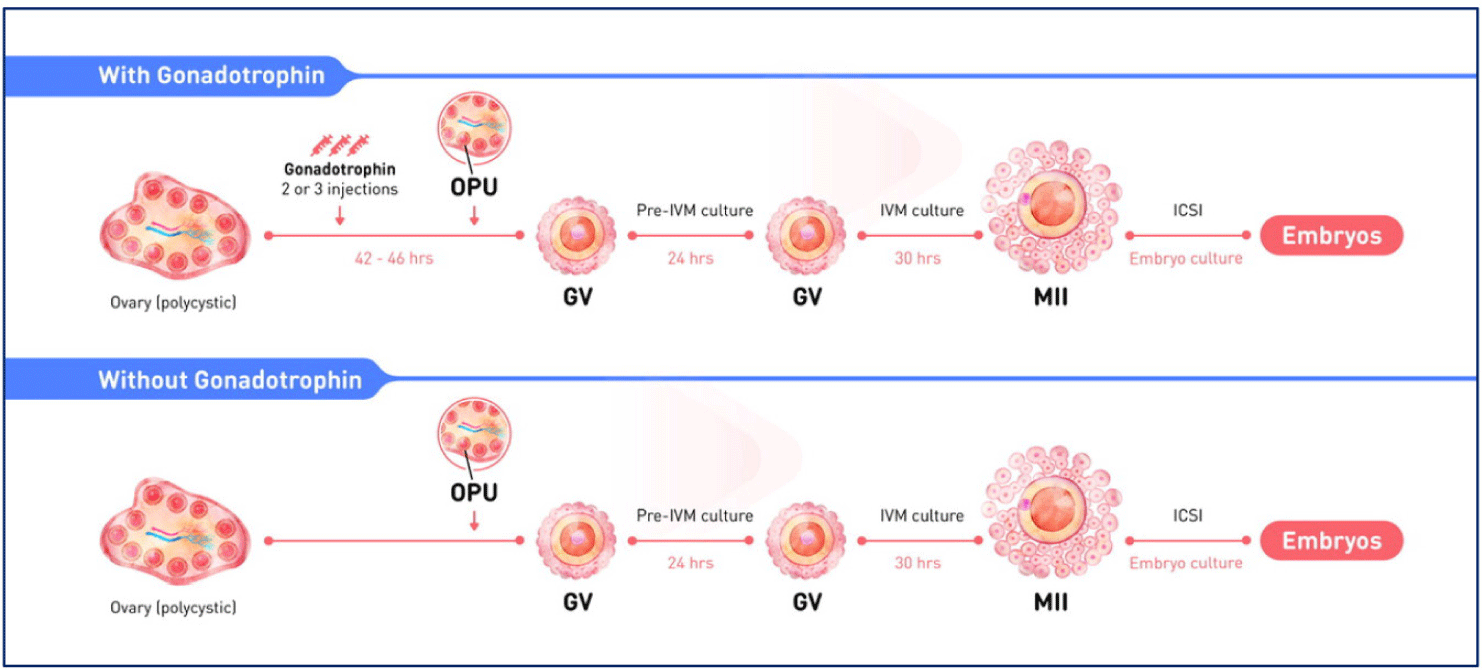
The biphasic IVM procedure consists of two phases. The first is a pre-IVM culture phase of 22–26 hours. Intact immature COCs are cultured with CNP and estradiol to maintain meiotic arrest. This phase preserves the bidirectional communication between oocytes and CC, enhancing oocyte cytoplasmic maturation. The second is an IVM culture phase of 30–32 hours. Oocytes are cultured in conventional IVM media with supplements to complete nuclear maturation. A flowchart of the CAPA-IVM process by day is shown in Fig. 2.
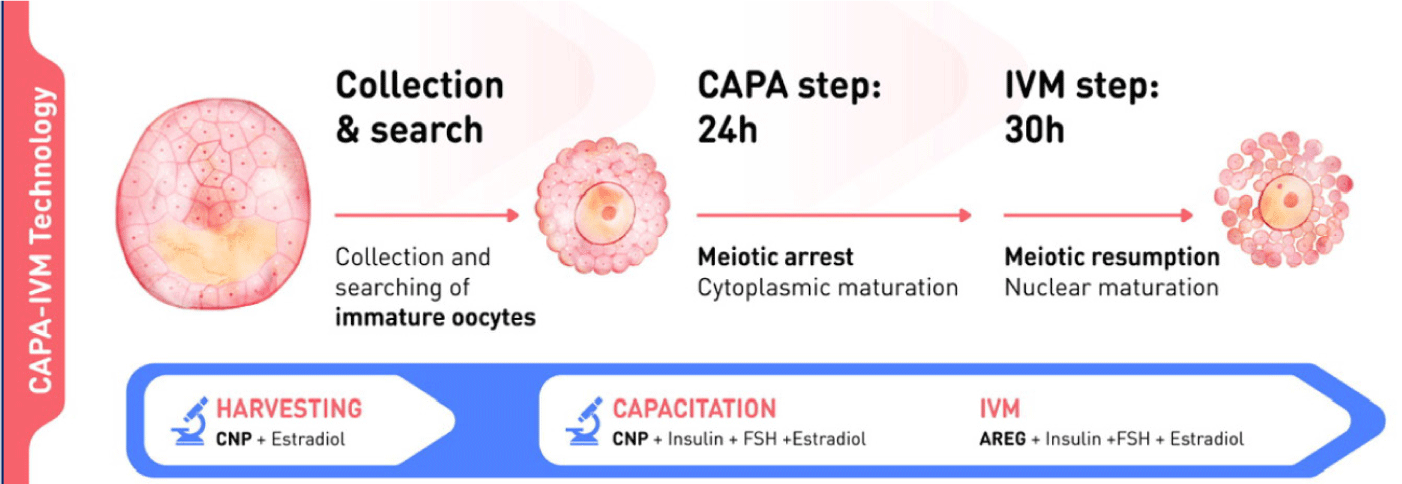
5. CLINICAL OUTCOMES USING BIPHASIC IN-VITRO MATURATION
Case numbers and main outcomes from the clinical application of biphasic (CAPA) IVM are summarised in Table 1. To date there have been eight trials that have studied biphasic IVM, with a total of 483 pre-IVM cycles [4,9,11–16]. Six of these studies were performed in the same centre – IVFMD, My Duc Hospital, Ho Chi Minh City, Vietnam – which is the only centre to have reported live birth outcomes after the use of any form of pre-IVM. There have been three preclinical safety and efficacy trials generating human embryos without embryo transfer; one using IBMX-mediated pre-IVM [17] and two using CNP-mediated pre-IVM [4,18].
| Study | Patients | Stimulation, mean FSH dose/patient, IU | Cycles, n1) | COCs, n1) | MII rate (%) | Good quality embryos (%) | Embryos transferred (n, mean) | Clinical pregnancy rate (%) | Live birth rate (%) | Live births, n1)2) |
|---|---|---|---|---|---|---|---|---|---|---|
| Sánchez et al., 2017 [4] | PCOS | 680 | 15 | 117 | 70 | 433) | NA | NA | NA | NA |
| Sánchez et al., 2019 [13] | PCOS+high AFC | 450 | 20 | 305 | 62 | 243) | NA | NA | NA | NA |
| Vuong et al., 2020 [9] | PCOS+high AFC | 379 | 40 | 700 | 64 | 193) | 2.0 | 60 | 47 | 19 |
| Vuong et al., 2020 [14] | PCOS+high AFC | 373 | 268 | 3,806 | 64 | 213) | 1.9 | 51 | 35 | 120 |
| Vuong et al., 2021 [15] | PCOS+high AFC | 300 | 40 | 732 | 67 | 233) | 2.0 | 35/704) | 20/604) | 16 |
| Akin et al., 2021 [11] | PCOS+high AFC | 300 | 30 | 555 | 67/555) | 20/153)5) | 1.9 | 67/435) | 47/295) | 11 |
| Kirilova et al., 2021 [12] | Gynaecological malignancies | 0 | 10 | 1056) | 56 | NA | NA | NA | NA | NA |
| Vuong et al., 2025 [16] | PCOS | 0 | 60 | 1,200 | 65 | 33.37) | 1.05 | 43.3 | 38.3 | 42 |
| Total | 483 | 7,520 | 208 |
6. SAFETY OF BIPHASIC IN-VITRO MATURATION
Biphasic IVM has mostly been used in women who are at higher risk, or are more difficult to treat, with conventional IVF. In particular, this group includes women with PCOS or a high AFC, who are at increased risk of exaggerated ovarian response, ovarian hyperstimulation syndrome (OHSS), ovarian torsion and the risks associated with high steroid hormone concentrations after ovarian hyperstimulation.
No cases of OHSS were recorded in any of the five recent trials using biphasic IVM, over a total of 483 cycles and using 2–3 days of FSH priming or no FSH priming before oocyte retrieval [9,11,13–16].
In a small randomized controlled trial (RCT) comparing biphasic IVM and standard IVM that included follow-up to live birth [14], clinical pregnancy, live birth, miscarriage, ectopic pregnancy, preterm delivery were all similar between the two different types of IVM. The results of a larger RCT comparing biphasic IVM (CAPA-IVM) with conventional ovarian stimulation and IVF up to live birth also provide reassurance regarding the safety of biphasic IVM [9]. There were no significant differences between the CAPA-IVM and IVF groups with respect to the occurrence of pregnancy complications, obstetric and perinatal complications, preterm delivery, birth weight and neonatal complications [9]. Two-year follow-up of children born to participants in this RCT showed that overall development up to 24 months of age was comparable in those born after CAPA-IVM or IVF [19].
In a prospective cohort study in Vietnam, children born after biphasic IVM were propensity score-matched with those born after natural conception and followed up to a maximum of 24 months [20]. The mean age of children at the end of follow-up was 15 months. The proportions of babies with any abnormal Ages & Stages-3 (ASQ-3) score or with any developmental red flag were not statistically different between children from the biphasic IVM group and those conceived naturally [20]. Although current data are limited, the available evidence suggests that the use of biphasic IVM does not have any negative effect on childhood physical and mental development.
7. FUTURE CLINICAL INDICATIONS FOR BIPHASIC IN-VITRO MATURATION
Given the effectiveness, safety, patient convenience and compliance with biphasic IVM, along with the good progress in improving pre-IVM culture systems, it is worth considering whether this approach has additional applications beyond its current clinical use primarily in women with PCOS (Table 2). A significant level of IVM expertise at the center and for the clinician is required to successfully use biphasic IVM for less common indications such as ovarian resistance to gonadotropins or ovarian auto-immunity. Such expertise can only be gained by a center/clinician routinely performing biphasic IVM on less difficult cases, such as women with PCOS and/or a high AFC. There is significant new interest in the application of biphasic IVM in onco-fertility (for both ex vivo ovarian tissue and in vivo oocyte pick-up [OPU])-collected oocytes, and we can expect a significant uptake of pre-IVM culture methodologies for this scenario. Due to its simplicity and efficacy, and the fact that women undergoing IVM can travel with certainty very soon after OPU because there is no risk of OHSS [21], one potential future application of biphasic IVM could be for tourism-fertility treatment, or to treat infertile couples in the context of pandemics like COVID-19. Similarly, due to its mild approach, convenience and being able to schedule an OPU date with certainty, low- or zero-stimulation forms of IVM using a biphasic IVM protocol may be attractive to clinics and patients for social egg freezing.
8. CONCLUSION
Biphasic IVM represents a promising advancement in assisted reproductive technology, offering a safe and potentially more effective alternative to conventional IVM. Its applications in PCOS, women with high AFC, normo-responders, gonadotropin-ROS, and fertility preservation highlight the versatility of biphasic IVM and its potential to improve clinical outcomes. Further research and clinical trials will continue to refine biphasic IVM protocols and expand indications for its use, making biphasic IVM a valuable novel tool in reproductive medicine.
