1. INTRODUCTION
There is a growing body of evidence showing that infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can have negative effects on physical and mental health that persist for more than three months [1]. This condition is known as “long COVID” or post-COVID-19 syndrome (PCS). Affected individuals report a variety of symptoms, including fatigue, chest pain, decreased exercise capacity, cognitive impairment, dyspnea, fever, headache, ageusia, anosmia, tachycardia, palpitations, and other symptoms [1]. Current available data from around the world indicate that 15–76% of people with coronavirus disease (COVID-19) develop persistent symptoms that last for at least six months. Of these, up to 80% of patients need medical attention within two months of recovering from the disease [1].
In Vietnam, there was a significant peak of SARS-CoV-2 infections from June to September 2021. Over this period, a community-based COVID-19 care model was implemented in District 10 of Ho Chi Minh City. The program involved two teams with distinct responsibilities. Team 1 was responsible for remotely monitoring individuals with COVID-19 who were isolating at home and providing referral and triage of acutely ill patients. Moderate and severe acute COVID-19 individuals from team 1 needed to be transferred to team 2, which was called Temporary Emergency Center, for additional care. The center only operated from July 27 to September 15, 2021, before concluding its mission [2].
The aim of the current study was to investigate health issues in patients who had been hospitalised for the treatment of moderate-severe COVID-19 and then subsequently recovered from acute SARS-CoV-2 infection.
2. METHOD
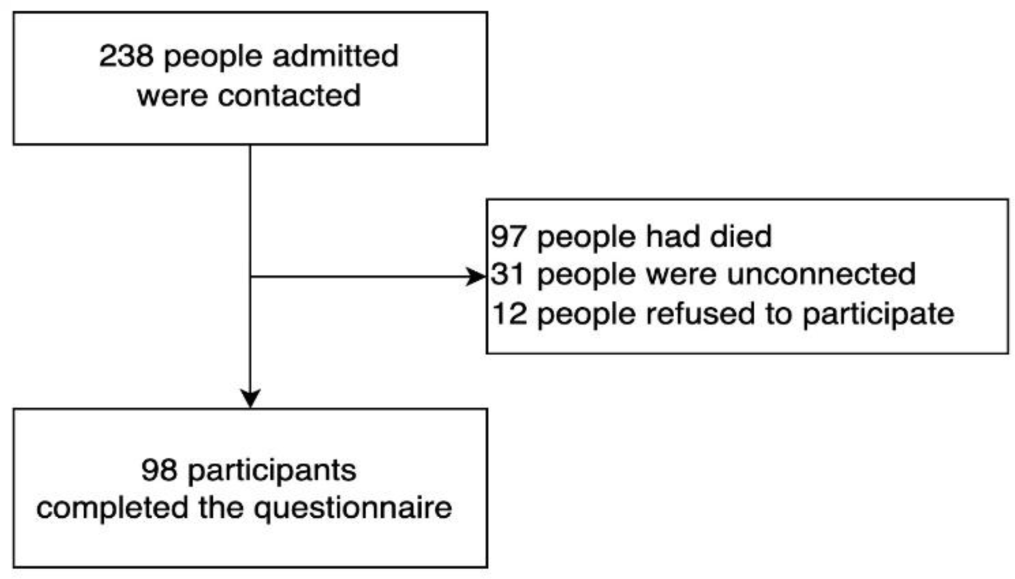
This cross-sectional study included all individuals with moderate-severe COVID-19 who had been hospitalised at the Temporary Emergency Center in District 10, Ho Chi Minh City, Vietnam between July 27 and September 15, 2021. The inclusion and exclusion criteria were below. A detailed study flowchart is shown in Figure 1.
Inclusion criteria:
-
- COVID-19 patients were admitted to the Temporary Emergency Center in District 10, Ho Chi Minh City during the period from July 27, 2021 to September 15, 2021.
-
- COVID-19 patients with moderate or severe triage according to No. 3416/QÐ-BYT on the Guidelines for the Diagnosis and Treatment of COVID-19 caused by the new coronavirus strain (SARS-CoV-2) of Vietnamese Ministry of Health.
-
- COVID-19 patients who have completed treatment and been discharged from the hospital.
Exclusion criteria:
-
- Patients who died during or after COVID-19 treatment for any reason during the study period.
-
- Patients who could not be contacted.
-
- Patients who refused to participate in the study.
-
- Patients who were uncooperative during the interview or had difficulty collecting information.
-
- Patients with psychiatric disorders, cognitive impairment, and hospitalized patients due to other illnesses.
All participants were contacted via telephone for data collection during the 12th week following their admission to the Temporary Emergency Center, as recorded in their medical records. A questionnaire was used to collect data on post-COVID-19 symptoms (see below for details). Data stored in medical records was also used to collect obtain relevant demographic, clinical and health information.
The acute phase of COVID-19 describes the symptoms of COVID-19 that occur within the first 4 weeks from the onset of symptoms. The subacute phase refers to symptoms that persist beyond 4 to 12 weeks after initial symptoms occur. Post-COVID-19 syndrome has defined as a status in which symptoms that appear during or after an acute COVID-19 infection lasted for more than 12 weeks, and are not attributable to any other medical condition [3]. The definition of health issues after moderate-severe COVID-19 is similar to post-COVID-19 syndrome, but it should be noted that the findings were based on a low response rate of participants and certain assumptions.
A questionnaire used in the study was developed based on common post-COVID-19 symptoms that had been previously reported [1]. It was tested in a pilot study that included 20 adults who had recovered from COVID-19, and then revised based on the responses and suggestions obtained to generate the final questionnaire, which was provided in the online supplement.
The final questionnaire consisted of 24 questions divided into three sections: personal information, symptom characteristics during acute COVID-19 illness, and symptom characteristics in the post-COVID-19 period. The personal information section collected demographic and epidemiological characteristics such as address, weight, height, age, and COVID-19 vaccination history. The acute COVID-19 section focused on Center entry date, discharge date, comorbidities, and respiratory support technique during hospitalisation. The post-COVID-19 section of the questionnaire collected data on self-measured current vital signs, current general health, common post-COVID-19 symptoms grouped into seven different organ systems, and the timing of these symptoms, including onset, resolution, or persistence.
Continuous variables are presented as median (interquartile range, IQR), and categorical variables are presented as number and percentage. The Wilcoxon rank sum test, Pearson’s Chi-squared test, or Fisher exact test was used to compare differences between patients with and without sequelae, as appropriate. Logistic regression models were employed to determine factors associated with the occurrence of PCS. The variables included in the model were chosen based on their likelihood of being associated with PCS. All analyses were performed using R 4.1.2 software (R Foundation for Statistical Computing; https://www.r-project.org/). A P-value of <0.05 was considered statistically significant.
3. RESULTS
Out of the total of 238 individuals who were hospitalized at the Temporary Emergency Centre in District 10, only 98 (41.2%) individuals completed the questionnaire. The study flowchart indicates that 140 individuals were met the exclusion criteria of the study as shown in Figure 1. Among them, 97 patients had died, 31 patients could not be contacted (after at least 3 failed attempts via phone), so their health status was unknown. The remaining 12 individuals knew that their health status was good but refused to participate in the study. The median (IQR) age of the study population of 61.0 (51.0, 67.0) years, with just over one-third of them belonging to the age group of aged 60–69 years. Furthermore, nearly two-thirds (63.3%) of the participants were female, as indicated in Table 1. The median (IQR) time of contact after COVID-19 illness was 15 (14.0, 17.0) weeks.
Of the 98 individuals for whom data was available, 72 (73.5%) developed PCS, while 26 (26.5%) did not. The most common clinical symptoms experienced at the onset of COVID-19 illness were fatigue (40.8%), dyspnea (30.6%), and cough (11.2%), as shown in Table 1. More than three-quarters of patients had at least one comorbidity, with the most common of these being overweight (56.0%) and hypertension (42.9%), as indicated in Table 1. The number and type of comorbidities did not differ significantly between individuals with and without health issues after moderate-severe COVID-19. During hospitalization, supplementary oxygen via nasal cannula or face mask (77.6%) was the most commonly used respiratory support, followed by high-flow nasal cannula oxygen therapy (HFNC), non-invasive ventilation (NIV) or invasive mechanical ventilation (IMV) required by 18.4% of patients (Table 1). As expected, those who developed health issues after moderate-severe COVID-19 were significantly more likely to report that their health was worse than before compared to those who did not develop (Table 1).
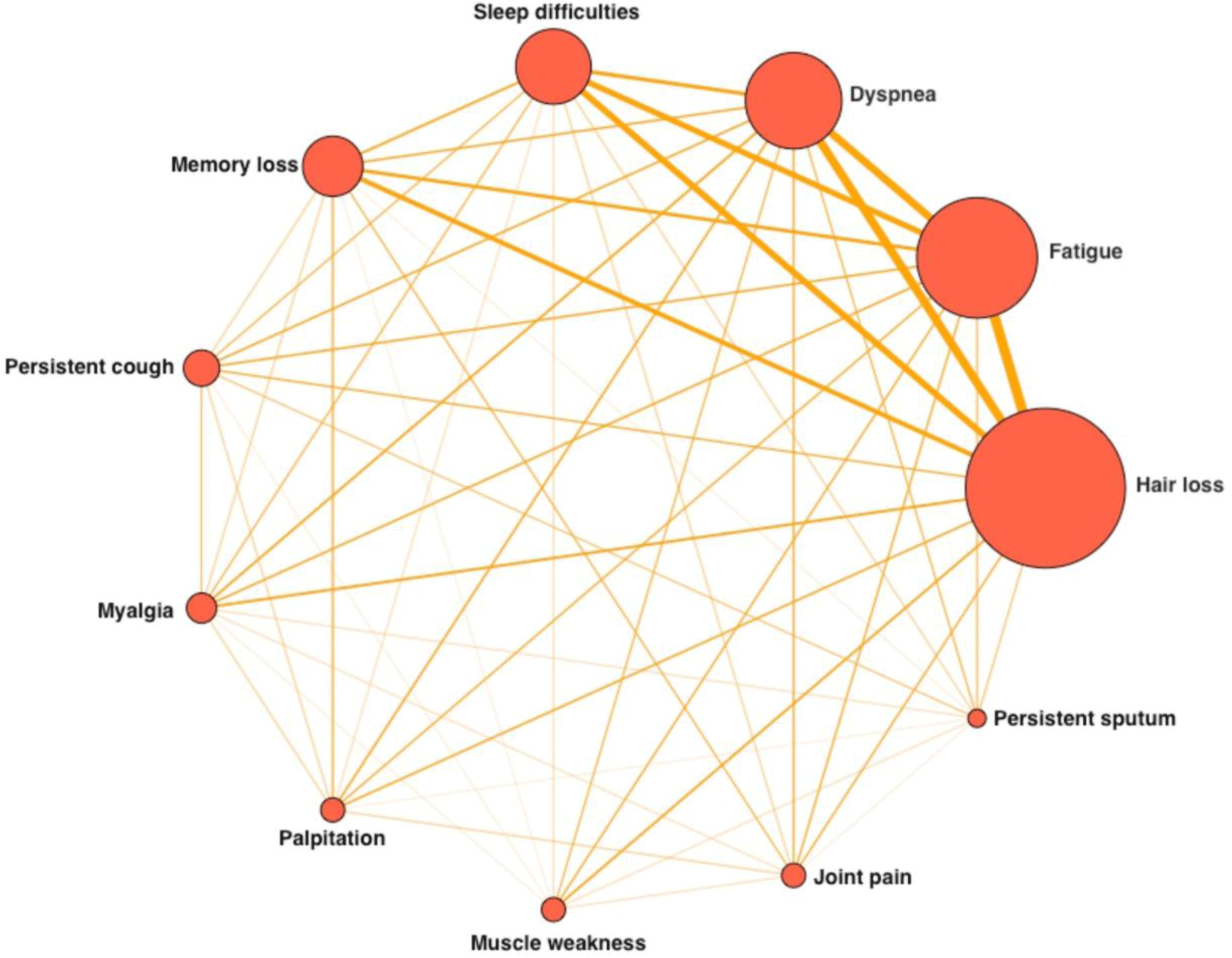
The complete details of symptoms reported during the acute and subacute phases of COVID-19 illness, as well as those of health issues after moderate-severe COVID-19, are presented in Table 2. The most common symptoms reported were hair loss, fatigue, dyspnea, sleep difficulties, memory loss and persistent cough, and these symptoms were consistent across different phases of the disease. The rates of these symptoms in individuals with health issues after moderate-severe COVID-19 were 56.1%, 42.9%, 33.7%, 26.5%, 20.4%, and 12.2%, respectively, as shown in Table 2. Hair loss was found to be a significant symptom after moderate-severe COVID-19, and it was more commonly reported in younger patients and those with a body mass index (BMI) ≥25 kg/m2. However, the frequency of fatigue, dyspnea, and sleep difficulties did not differ significantly based on age, BMI, or respiratory support technique during hospitalization, as indicated in Table 3. The frequency and associations between different symptoms after moderate-severe COVID-19 are visually represented in Figure 2.
On binary logistic regression analysis, the only significant potential contributing factor for the development of health issues after moderate-severe COVID-19 was the highest respiratory support technique used during hospitalization. Compared to individuals who required HFNC, NIV or IMV, those who only needed supplemental oxygen via nasal canula, or face mask were at significantly lower odds of developing health issues after moderate-severe COVID-19 (odds ratio 0.13, 95% confidence interval 0.01–0.68, P=0.049) (Table 4).
4. DISCUSSION
This is the first report describing health issues in patients who have recovered from moderate-severe COVID-19 patients in Vietnam. Since we only conducted our study on moderate to severe COVID-19 patients, as per the guidelines of the Vietnamese Ministry of Health, who have required care in hospitals or specialized medical facilities. This was also the function of our Emergency Center. Furthermore, our research objectives aimed to investigate the proportion and clinical characteristics of this population, as at that time there were limited data on health issues after moderate-severe COVID-19, including both in Vietnam and worldwide. This limited data also applied to the understanding of post-COVID-19 syndrome (PCS). We found that 73.5% of patients with moderate-severe COVID-19 were still symptomatic approximately 3 months after the acute illness phase. This prevalence is quite similar to several published articles at 50– 70% of hospitalized cases. The median age of our study population is quite high, which may explain why our health issues after moderate-severe COVID-19 rate is slightly higher compared to other studies. Meanwhile, the PCS incidence is estimated at 10–30% of non-hospitalized cases [4]. The findings of the study suggest that a significant proportion of individuals who had experienced moderate to severe COVID-19 continue to experience at least one symptom even after a certain time period. The four most frequently reported symptoms among these patients were fatigue, hair loss, shortness of breath, and sleep difficulties. Importantly, these symptoms were consistent with those observed during the acute and subacute phases of COVID-19 illness. Furthermore, the study identified that the need for respiratory support using HFNC, NIV, or IMV during the initial hospitalization period was the significant potential contributing factor for developing health issues after moderate-severe COVID-19. This suggests that the severity of respiratory support needed during hospitalization may play a role in the development of health issues in patients recovering from moderate to severe COVID-19.
After the second wave of COVID-19 in Vietnam, there was a significant increase in the number of COVID-19 cases and the number of recovered patients. Although factors influencing prognosis and mortality during COVID-19 illness have been extensively documented in the literature, there is limited information about health issues after COVID-19, particularly after moderate-severe COVID-19 illness. Our findings that fatigue and sleep difficulties were among the most prevalent symptoms after moderate-severe COVID-19 are consistent with published literature [5, 6].
Fatigue is a general symptom that is hard to describe correctly. It could be a sign of abnormalities in many different organ systems and could overlap with other symptoms such as dyspnea, and other psychological, neurological, and cognitive disorders. In our study, fatigue symptoms were often accompanied by other multi-organ symptoms, similar to a previous study by Halpin et al that included 100 patients previously hospitalised with COVID-19 [5]. Fatigue is also a common persistent symptom in patients who have had other viral infections such as severe acute respiratory syndrome (SARS), Ebola, and influenza H1N1 [7, 8].
Sleep difficulties was another common symptom in our study, reported by 26.5% of individuals after moderate-severe COVID-19. Studies by Carvalho-Schneider and Moreno-Perez et al also showed that sleep difficulties were frequent in PCS [9, 10]. It has been suggested that this symptom may be due to the effects of SARS-CoV-2 on neurons that express angiotensin-converting enzyme receptor 2 (ACE2). Through binding with ACE2, the coronavirus can penetrate both the central nervous system and peripheral tissues, thereby causing respiratory insufficiency through neuroinvasion in the brainstem, which is responsible for regulating cardiorespiratory function. Because there exists a close association between breathing disorders and sleep, the resulting neurological impairment can lead to long-lasting effects on respiratory regulation, potentially worsening the quality of sleep in post COVID-19 patients [11].
In the current study, the rate of hair loss was higher in individuals with a younger age or higher body mass index, without any difference in males versus females. This differs from the findings of a systematic review that identified female sex as a risk factor for hair loss after COVID-19 [12]. The pathogenesis of hair loss in this setting is still not fully understood, but it has been hypothesised that interleukin-6, a cytokine that is abundantly released in patients with severe COVID-19, causes hair growth inhibition [13].
Persistent dyspnea was also a frequent symptom after moderate-severe COVID-19 in our study, occurring in 33.7% of patients, even though patients were determined to have fully recovered from COVID-19 illness. According to our data, of the eighteen patients, two had underlying respiratory conditions and sixteen had cardiovascular diseases, only ten of the cardiovascular group presented with dyspnea after contracting SARS-CoV-2. Eight of these patients continued to experience dyspnea during the post-COVID-19 phase. Therefore, it can be inferred that dyspnea is likely a result of SARS-CoV-2 infection. However, COVID-19 may exacerbate the pre-existing conditions, particularly in the cardiovascular and respiratory systems. Consequently, dyspnea may be a result of the overlap between the progression of underlying medical conditions and the post-COVID-19 status. We did not find any significant associations between dyspnea and other potential contributing factors in our population. In contrast, a UK-based study found that patients with COVID-19 who had been admitted to the intensive care unit for respiratory support and experienced breathlessness after hospital discharge had a history of prior lung disease, were older and had a high body mass index [5]. The cause of dyspnea in individuals with PCS is not fully understood, but potential mechanisms include fibrous proliferation of damaged alveoli caused by cytokines such as interleukin-6 and transforming growth factor-β [14], or diaphragm dysfunction [15]. In addition, pulmonary interstitial changes on multi-slice computed tomography have been observed 6 months after the onset of COVID-19 illness [16].
In our study, only 3% of individuals after moderate-severe COVID-19 reported loss of smell. This contrasts with systematic review article data, where up to 26% of patients were experiencing anosmia at 3 months after COVID-19 illness [1]. The reasons for this marked difference in prevalence are unclear. One contributing factor may be differences in population characteristics, but this needs to be studied further.
It has been hypothesized that PCS might be caused by mast cell activation syndrome (MCAS) [17]. Improper mast cell activation leads to a large increase in mediators, causing multi-organ pathology, including cardio-pulmonary, gastrointestinal, dermatological, and neurological symptoms [18]. Additional studies are needed to investigate whether there is a close association between MCAS and PCS. If confirmed, this could allow the pharmacological blockade of relevant mediators, including histamine, leukotrienes, and prostaglandin, or inhibition of mediator synthesis, as a prophylaxis and/or treatment for PCS [19].
Our finding that patients who require more intensive respiratory support are at higher potential of developing PCS is consistent with published studies showing that the need for intubation or intensive care unit admission was associated with many persistent symptoms [5, 20, 21]. In contrast, other studies have not shown any association between the severity of the acute phase of COVID-19 illness phase and PCS [22-24]. In another systematic review and meta-analysis, Maglietta et al identified female sex and the severity of the acute COVID-19 episode as risk factors for PCS [25]. Although our data support the latter of these, sex was not a potential contributing factor for health issues after moderate-severe COVID-19 in our population.
We did not provide direct help or treatment to patients with health issues after moderate-severe COVID-19 in this study. However, if we identified patients with persistent COVID-19 symptoms, they might have recommendation to seek medical advice and treatment from specialized clinics or hospitals that focus on the management of post-COVID-19 syndrome. The specific interventions or treatments these specialized facilities provide would likely depend on the individual patient’s symptoms and needs.
Our study collected data retrospectively and administered a questionnaire via telephone. This approach is appropriate for limiting direct contact during the COVID-19 epidemic. However, several limitations also need to be considered when interpreting our findings. First, although modified after initial testing, the questionnaire was specifically created for this study and was not externally validated. The responses to the questionnaire could have been influenced by recall bias and were subjective in nature, as they were based on patients’ experiences rather than objective medical diagnoses. In addition, the comparatively elderly nature of the study population introduces the possibility of the impact of cognitive decline. Another limitation is that follow-up took place at approximately 3 months after the acute phase of illness, meaning that we do not have any data on longer-term rates and manifestations of PCS. Moreover, caution should be exercised when generalizing the results of our study to the wider population due to the relatively low response rate of 41.2%. Consequently, the findings may not provide a comprehensive representation of individuals who have recovered from moderate-severe COVID-19. Additionally, the study’s specific focus on a particular population, predominantly elderly individuals from a single district in Ho Chi Minh City, Vietnam, may restrict the generalizability of the study’s findings to other populations of individuals who have recovered from moderate-severe COVID-19. Besides, we do not state any risk factor in this study. The study’s sample size is not large enough to find any good relationship. Univariate analysis result was not feasible. Therefore, we did not further analyze the multivariate model for risk factors. Our findings are only suggestive as potential contributing factors, not conclusive evidence of risk factors. We acknowledge that these variables may play a role in contributing to the development or exacerbation of health issues but do not imply a definitive causal relationship. This is only a secondary outcome in our study.
Despite these limitations, our study provides valuable insights into the self-reported health issues experienced by individuals who have recovered from moderate-severe COVID-19. The findings can serve as a basis for further research and highlight the importance of addressing the specific health needs of this population.
Conclusion
This analysis provides the first indication of the prevalence of health issues after COVID-19 in Vietnam, and documents the symptoms experienced by patients after moderate-severe COVID-19. These findings could assist the local health system in detecting and managing post-COVID-19 syndrome in the future. However, longer-term longitudinal observational studies are needed to fully elucidate the ongoing health consequences of COVID-19 illness.