1. INTRODUCTION
Stroke is one of the top causes of disability and death in Vietnam. The sequelae caused by stroke are extremely heavy, affecting patients’ quality of life, their families, and society [1]. Stroke rehabilitation is essential; if carried out comprehensively and correctly, it can cause an improvement in the quality of life and work productivity of the patients [2]. According to WHO, one of the most critical activities of stroke recovery is “Identifying functional difficulties and measuring them” and “Evaluating the effectiveness of interventions” using the standard measurement scales [3]. Thus, the scales are indispensable tools in the process of rehabilitation intervention, helping to measure the patient’s current condition or evaluate the outcome after treatment. These scales, which describe specific activity limits or functional levels and the overall performance of patients, can also be used to determine when they can be discharged from hospitals, compensation costs, and regulatory requirements [3]. This is extremely important in assessing the role and significance of treatment methods, especially Traditional medicine methods, in stroke recovery. In recent years, Traditional medicine has played an increasingly important role in post-stroke recovery, choosing an appropriate scale is a prerequisite in planning and evaluating the effectiveness of these interventions [4]. Many standardized and well-designed scales have recently been used to evaluate neurological deficits in post-stroke patients. These tools can comprehensively assess mobility and functional activities, among which the Fugl Meyer Assessment (FMA) [4], [6], [7], [8], [9], [10], [11], [12], Motricity Index (MI) [12], [14], [15], [16], [17] and Barthel Index (BI) [18], [19], [20], [21], [22] are widespread.
The FMA was first proposed by Axel Fugl-Meyer et al. in 1975 as a standard assessment test for stroke recovery [4]. It is widely used worldwide to assess motor function in clinical and research studies, describe motor recovery, and help clinicians plan and evaluate treatment outcomes. Before the advent of FMA, almost all motor rehabilitation and stroke outcome assessment methods were empirical or based on assessing ADL. Stroke rehabilitation professionals consider FMA one of the most comprehensive quantitative measurement tools [23], [24]. The MI is a scale of muscle strength of arms, legs, and trunk proposed by Demeurisse et al. in 1980 [13]; Collin and Wade developed its evaluation parameters in 1990 [14]. MI, related to the classification of muscle strength, is a reliable measure after a stroke that can be applied quickly and without special equipment or training [17]. Barthel Index (BI), published by Mahoney and Barthel in 1965, consists of 10 daily living activities (self-care and mobility), was developed to measure the level of disability in stroke patients with excellent intra and interrater reliability proven in many other studies [18], [19], [20]. In Vietnam, BI is one of the most commonly used tests to measure ADL in post-stroke patients, while MI and FMA have not been widely applied in research or clinical practice. Several studies suggested that BI, when used together with other scales (e.g., FMA), may have a complementary effect, supporting a clear assessment of the patient’s neurological deficits [25], [26]. Our study aims to evaluate the correlation between the FMA, MI, and BI scales, thereby serving as a basis for selecting appropriate scales for patients with hemiplegia after stroke.
2. MATERIALS AND METHOD
With a descriptive cross-sectional method, this study was conducted in 3 hospitals (HCMC Hospital of Traditional Medicine, University of Medical Center HCMC - Branch no.3, and HCMC Hospital for Rehabilitation and Professional Diseases) from February 2021 to January 2023. There were 128 hemiplegic patients after ischemic stroke who met all inclusion criteria and had no exclusion criteria. The researchers would collect essential data from study subjects such as pulse, blood pressure, temperature, breathing rate, BMI, and the duration from the onset of stroke.
Inclusion Criteria: All people who met all of the criteria: (1) diagnosis of cerebral infarction (based on standard criteria of Ministry of Health, or by medical record); (2) the duration from onset of stroke ranging from 24 hours to 3 months; (3) hemiplegia after stroke; (4) aged 18 years or more; (5) Barthel index ≤ 60. Exclusion Criteria: People were excluded if they met one or more of the exclusion criteria: (1) hemorrhagic stroke, (2) malignant tumors or infectious diseases; (3) did not stay awake or cooperate with the treating physician.
The main parameter evaluated in objects is the Barthel Index. BI was compared with the two parameters involving FMA-motor and MI. FMA-motor includes FMA-UE and FMA-LE. MI has MI-UE, MI-LE, and TCT (Trunk Control Test)
Barthel Index (BI) assesses the degree of independence in basic activities of daily living, including ten criteria: eating, bathing, cleaning the face, dressing, defecating, urinating, using the toilet, moving from bed to chair, walking on the floor, up or down stairs. The rating score is 0, 5, 10, or 15. The maximum total score is 100 (on a scale of 0-100), where 0 is entirely dependent, and 100 is completely independent [18].
Fugl-Meyer Assessment (FMA-motor) assesses motor function in a person with hemiplegia after stroke, in which FMA-UE assesses movement, coordination, and reflexes of parts including shoulder, elbow, forearm, wrist, and hand; FMA-LE assesses movement, coordination, and reflexes of the hips, knees, and ankles. The items on the scale are scored based on the ability to complete each item on a 3-point scale, where 0 = unable to perform, 1 = partially performed, and 2 = entirely performed. FMA-UE has 33 evaluation criteria with a maximum total score of 66, and FMA-LE has 17 with a complete total score of 34. The motor score of FMA (FMA-motor) ranges from 0 (complete paralysis) to 100 points (normal movement) [4].
The Motricity Index (MI) measures upper extremity, lower extremity, and trunk muscle strength. Movements for upper extremities: elbow flexion, shoulder abduction, pinch grip. Moves for lower extremities: knee extension, ankle dorsiflexion, and hip flexion. The score for pinch grip is 0 (No movement), 11 (Starting to grasp), 19 (Can grasp the cube but cannot hold it under the influence of gravity, the operator must raise the wrist), 22 (Can grasp and hold a cube under the force of gravity), 26 (Can grasp and hold a cube, overcome a slight pull, but still weaker than the opposite side), 33 (Normal muscle strength). Scores for the remaining actions are 0 (No movement), 9 (Feel muscle contraction, but cannot move), 14 (Movement cannot reach the full range of motion - ROM and cannot overcome gravity), 19 (Full range of motion and overcome gravity, but not resistance), 25 (Full ROM, overcome gravity but less strong than the other side), 33 (Normal muscle strength). The maximum score of the upper and lower limbs is 99 (scale from 0-99). TCT (Trunk Control Test) was assessed by sitting up from a lying position, turning to the weak side, turning to the strong side, and balancing in a sitting position. Rating score is 0 (cannot do), 12 (can only be done with non-muscular assistance such as pulling out the bed sheet or blanket, using hands to hold the body while sitting, pulling the monkey pole, etc.), 25 (Normal strength), the maximum total score is 100 (scale from 0-100) [20].
This study used SPSS 22.0 for data analysis. The format of means ± SD (standard deviation) expressed for data. In order to analyze the correlation between the scale scores, we used the Spearman correlation with a p-value < 0.05 meaning statistically significant. The correlation varies from 0.00 to 0.25; it shows no or weak relationship; from 0.25 to 0.50, it offers a small degree of relationship; from 0.50 to 0.75, it is moderate to good correlation; values above 0.75 means having excellent correlation [27].
3. RESULTS
Among 128 study subjects, the number of males was 73 (57%), that of females was 55 (43%), and the gender ratio was 1.33/1. The mean duration from the stroke onset was 31.76 ± 28.21 (days). The mean age of the participants was 61.09 ± 11.86 years old. Study subjects with hemiplegia of the dominant hand accounted for 68%, 2.12 times higher than those without paralysis of the dominant hand. The mean BMI was 23.14 ± 3.26, the mean SBP (systolic blood pressure) was 126.22 ± 11.17, and the mean DBP (diastolic blood pressure) was 78.96 ± 7.82 (Table 1).
The average scores of the scales are shown in Table 2. The Barthel Index had a mean score of 37.81 ± 16.69. Fugl Meyer Assessment motor (FMA-motor) had an average score of 28.18 ± 23.52, in which FMA-UE was 15.97 ± 16.60 and FMA-LE was 12.21 ± 8.18. The Motricity Index upper extremity (MI-UE) had an average score was 31.35 ± 28.05 and lower extremity (MI-LE) was 34.06 ± 21.62 while trunk (TCT) was 54.97 ± 31.84.
| Scales | Mean±SD (n = 128) |
|---|---|
| BI | 37.81 ± 16.69 |
| MI-total | 120.38 ± 73.17 |
| MI-UE | 31.35 ± 28.05 |
| MI-LE | 34.06 ± 21.62 |
| TCT | 54.97 ± 31.84 |
| FMA-motor | 28.18 ± 23.52 |
| FMA-UE | 15.97 ± 16.60 |
| FMA-LE | 12.21 ± 8.18 |
Abbreviations: BI: Barthel Index, MI-total: Total score of Motricity Index (MI-UE, MI-LE, TCT), MI-UE: Motricity Index Upper Extremity, MI-LE: Motricity Index Lower Extremity, TCT: Trunk Control Test, FMA-motor: Fugl-Meyer Assessment of Motor function, FMA-UE: Fugl-Meyer Assessment Upper Extremity, FMA-LE: Fugl-Meyer Assessment Lower Extremity.
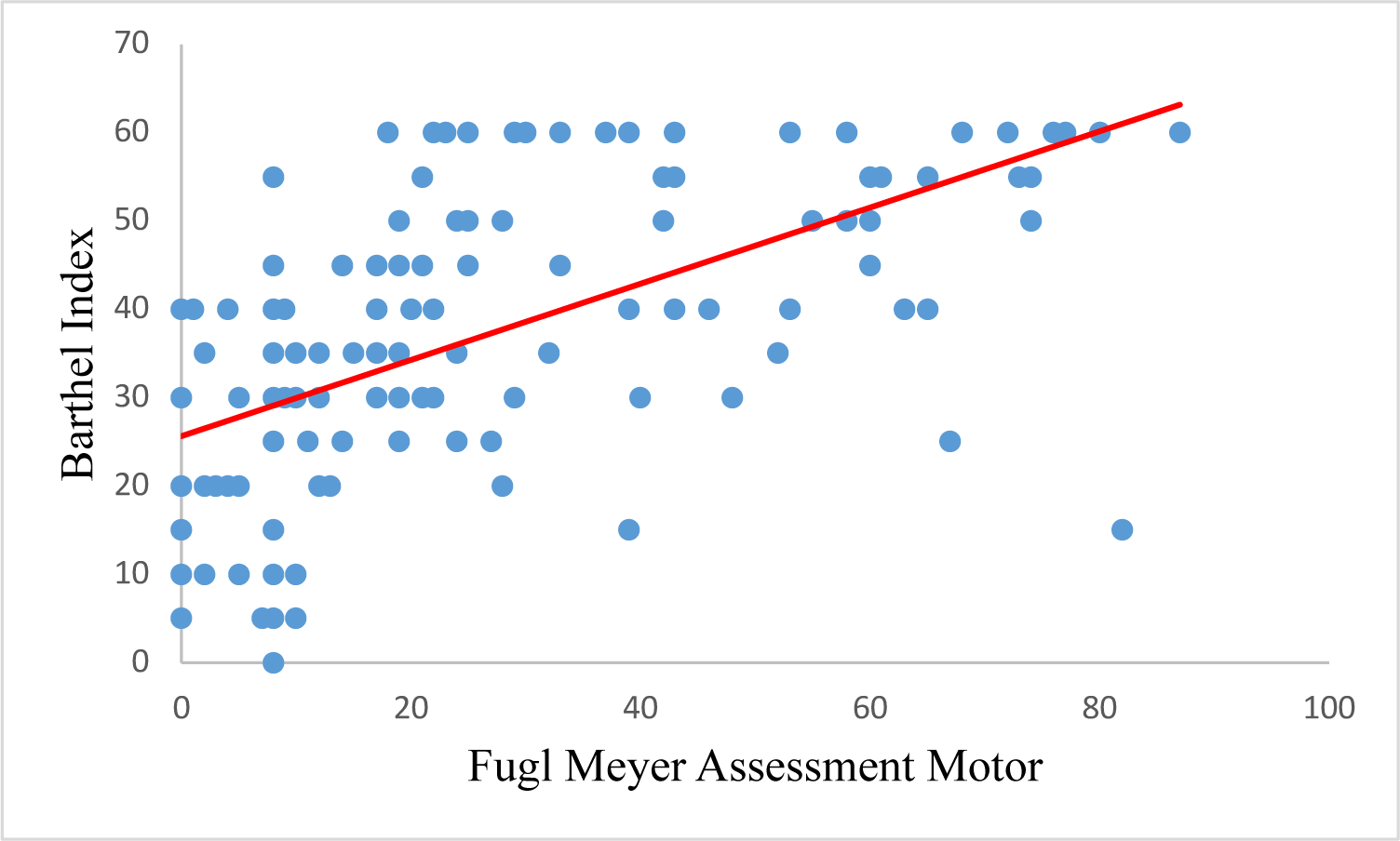
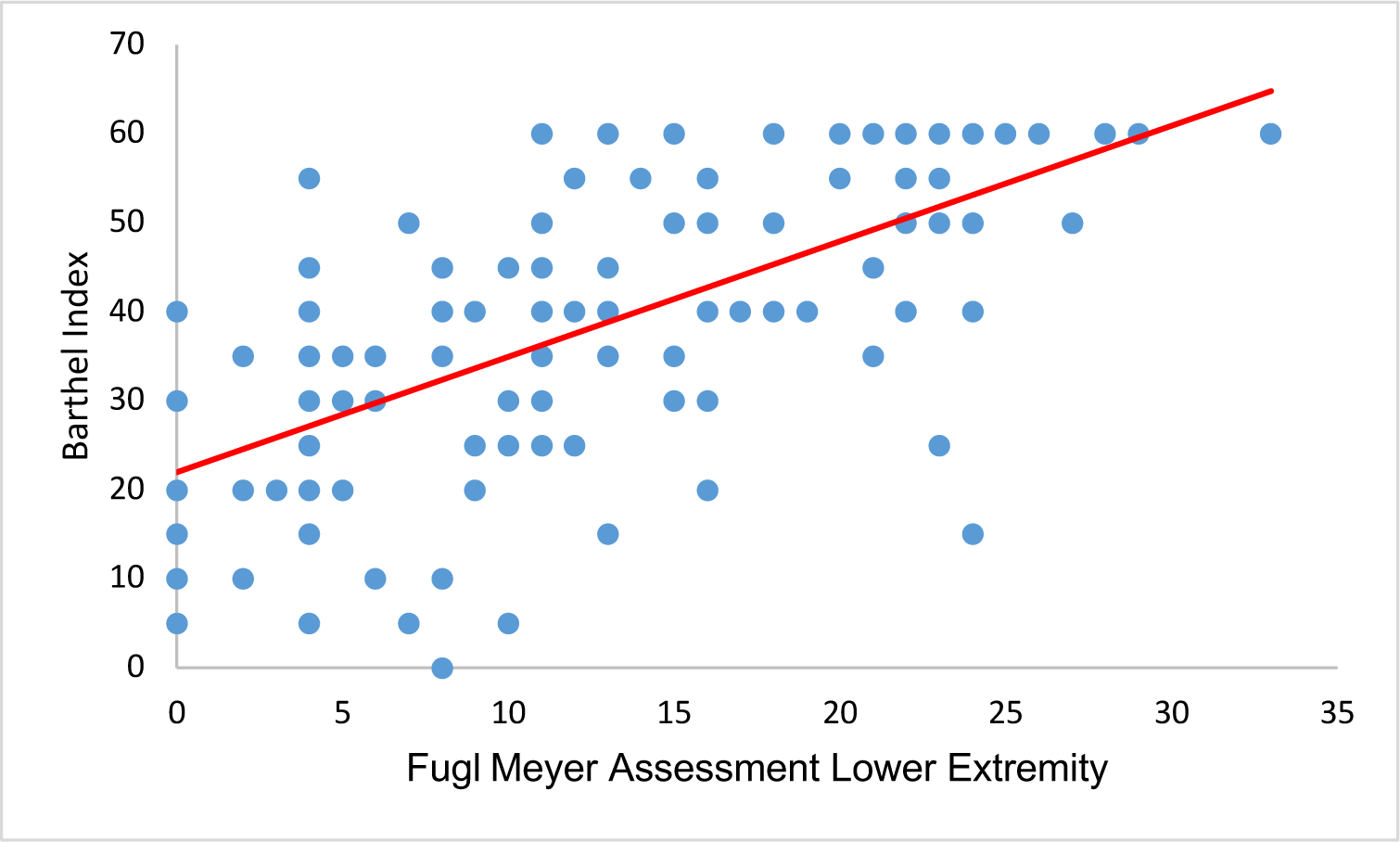
A significant correlation between the total FMA-motor and Barthel Index scores was found with r = 0.659 using Spearman’s correlation test with p-value < 0.05 (Table 3). The graph also showed the correlation between the FMA-motor and Barthel Index scales, whereby the higher the FMA-motor score, the higher the Barthel score (Figure 3). When analyzing the Spearman correlation in the FMA-motor sub-indices (FMA-UE and FMA-LE), data showed significant correlations between FMA-LE, FMA-UE, and Barthel Index, with coefficients correlation, 0.641 and 0.618, respectively (Figure 1 and Figure 2).
| Correlation Coefficient (r) | p-value (Spearman’s correlation) | |
|---|---|---|
| FMA-UE | 0.618 | 0.000 |
| FMA-LE | 0.641 | 0.000 |
| FMA-motor | 0.659 | 0.000 |

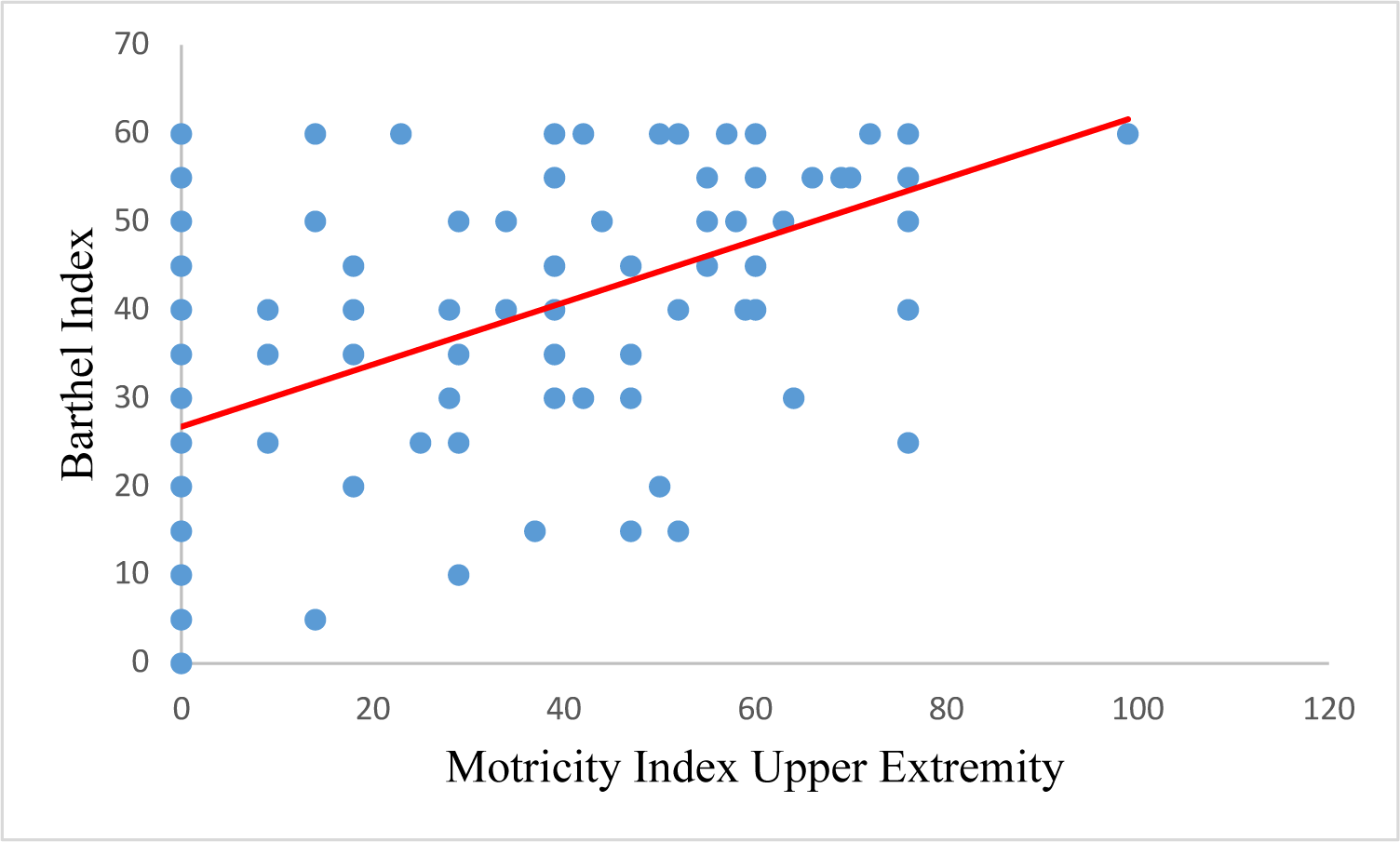
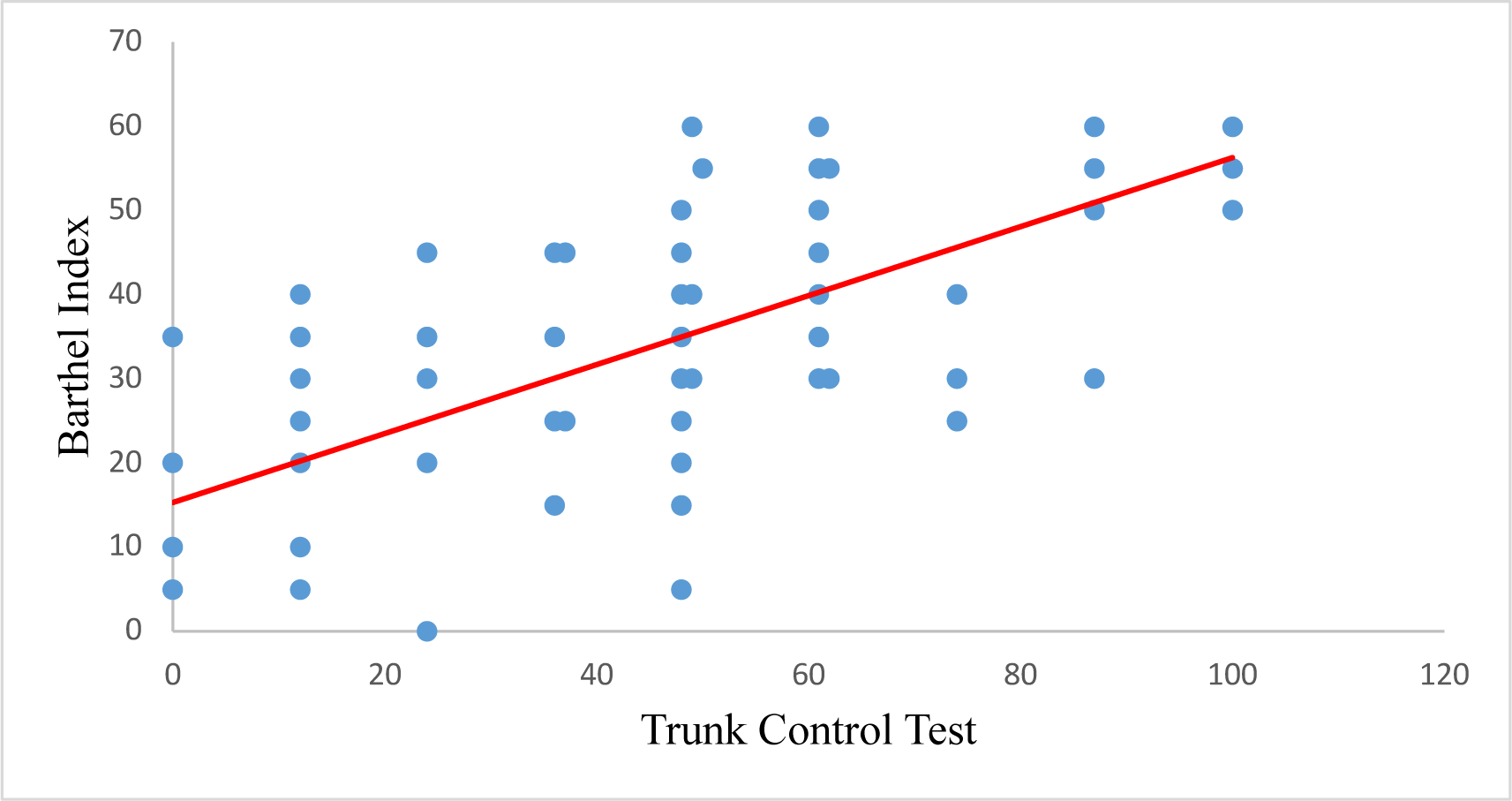
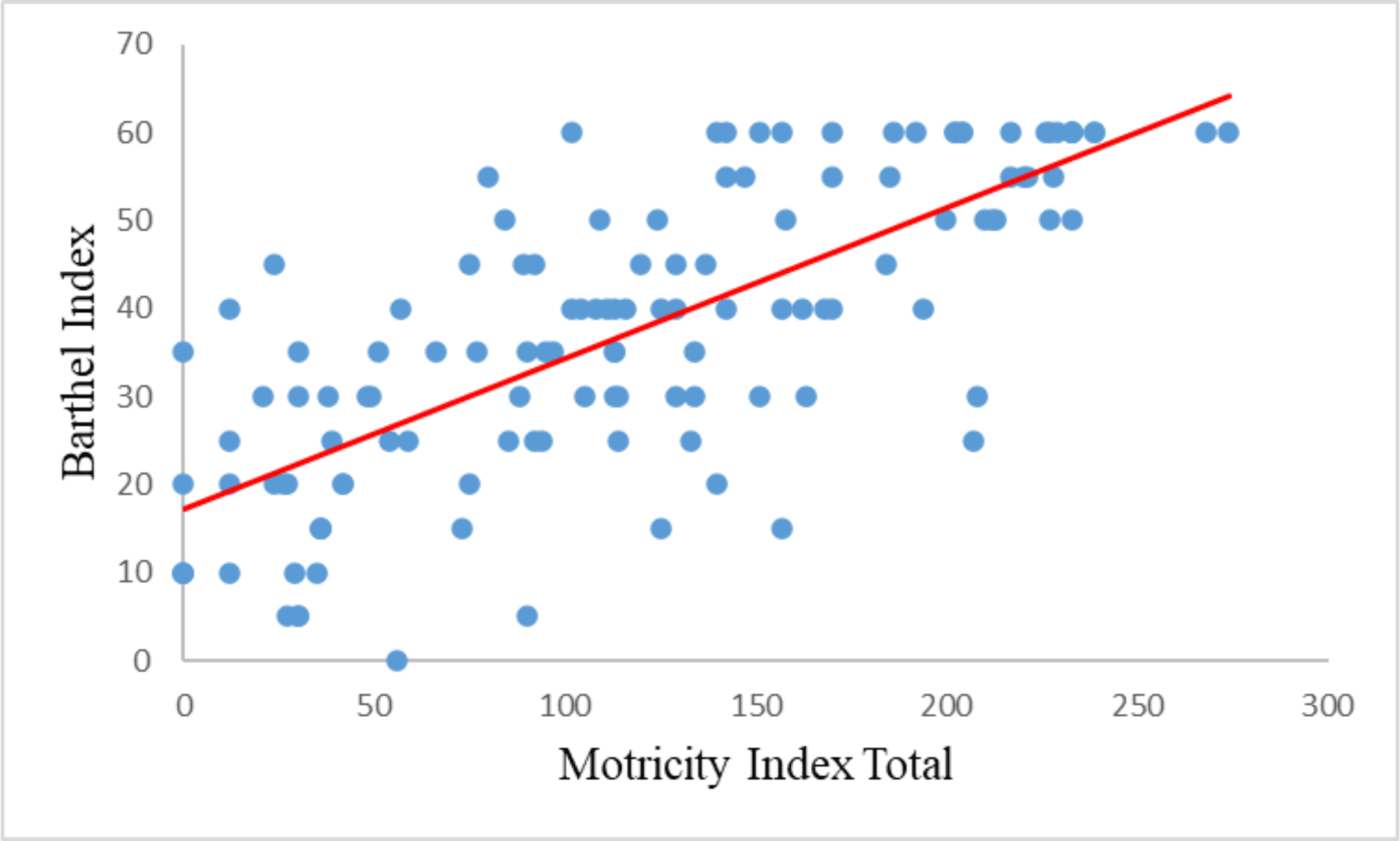
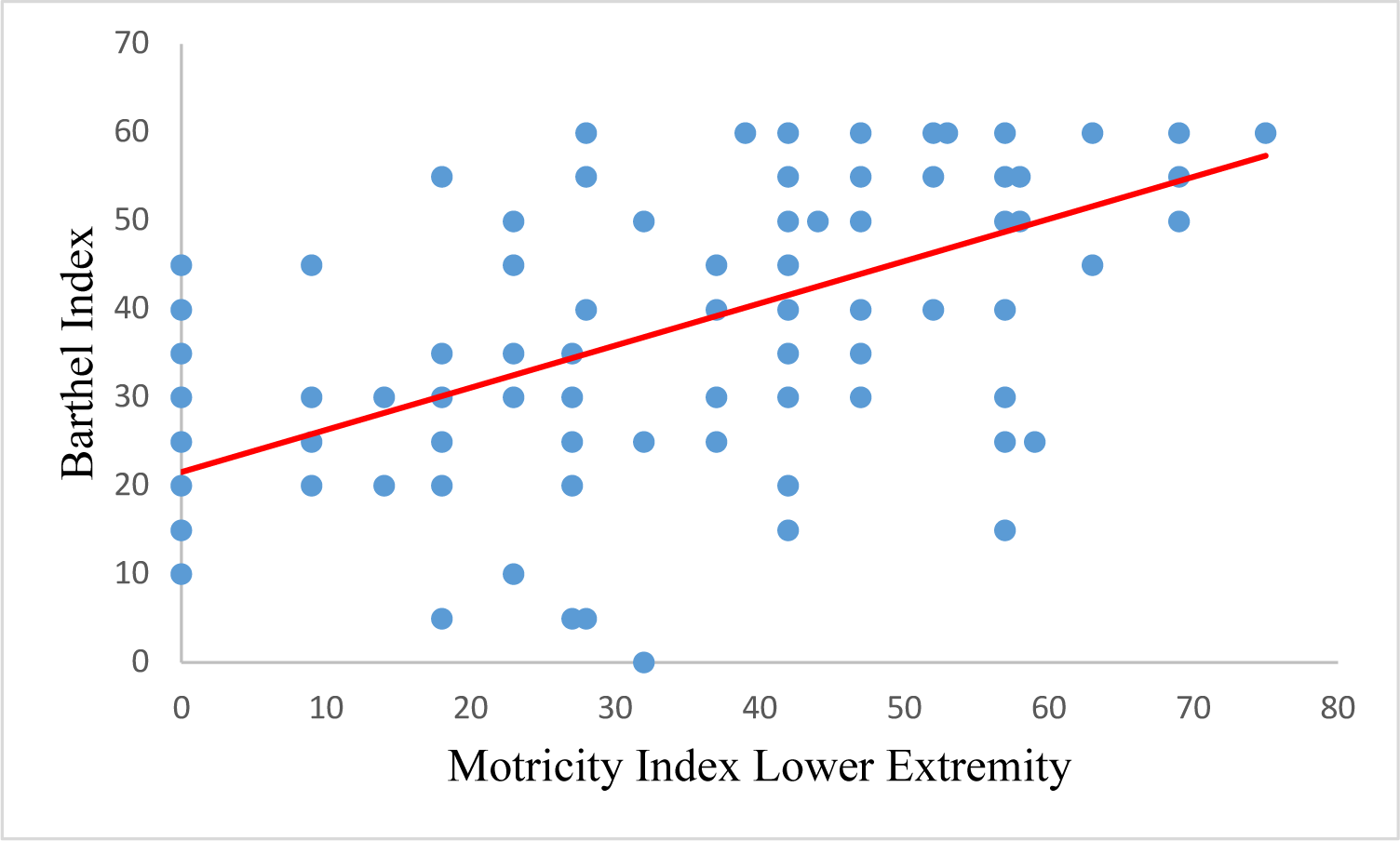
Spearman’s correlation analysis showed a significant correlation between the Motricity Index and the Barthel Index, with a correlation coefficient of 0.748. Subdomains of the Motricity Index (MI-UE, MI-LE, TCT) are also strongly correlated with the Barthel index with correlation coefficients of 0.596, 0.632, and 0.794, respectively (Table 4).
| Correlation Coefficient (r) | p-value (Spearman’s correlation) | |
|---|---|---|
| MI-UE | 0.596 | 0.000 |
| MI-LE | 0.632 | 0.000 |
| TCT | 0.794 | 0.000 |
| MI total | 0.748 | 0.000 |
4. DISCUSSION
The assessment of functional activity using scales, a crucial part of motor rehabilitation after stroke, can help physicians determine the severity of health problems and set appropriate goals as well as interventions for the recovery process. In Traditional medicine, choosing a scale suitable for research purposes is a prerequisite for proper intervention methods. According to the ICF, the assessment of functional activities includes assessments of body functions and structures, activities, and participation [28]. While Barthel is often used to evaluate activities or participation, FMA and MI are often used to assess body functions and structures [29].
In our study, the age of participants was 61.09 ± 11.86. This result is similar to Tae-Lim Kim’s study (53.6 ± 16.1) [29] and Husnul Mubarak’s study (55.9 + 10.58) [26]. The gender distribution in our study was male/female is 1.33/1, similar to that of Tae-Lim Kim’s study (1.38/1) [29]. The mean stroke time (duration from the onset) in our study was 31.76 ± 28.21 (days), which was shorter than that of Husnul Mubarak’s study (10.6 ± 7.95 months) and Oliveira’s study (3.63 ± 3.02 years). This difference may be because our research was on subjects with early recovery phase stroke (from 24 hours to 3 months), while Husnul Mubarak’s study was conducted on stroke subjects ≥ 3 weeks; the study of Oliveira observed stroke patients from 6 months to 15 years [25], [26].
Our study results showed that the average Barthel score was 37.81 ± 16.69 points, which was lower than that of Husnul Mubarak’s study, with the average Barthel score of 80.75 ± 16.56 points, ranging from 40-100 points, and Oliveira’s survey with the average Barthel score of 90.75 ± 5.45 [7]. Our study selected patients with functional impairment with a Barthel score of less than 60 (moderate, severe, and total dependence), while Husnul Mubarak and Oliveira’s study selected all post-stroke patients without classification levels of dependency (ADL). This study also revealed that the average FMA-motor score was 28.18 ± 23.52, FMA-UE was 15.97 ± 16.60, and FMA-LE was 12.21 ± 8.18. This result is also lower than that of Husnul Mubarak with FMA-UE of 43.08 ± 18.38 (4-66), FMA-LE of 22.55 ± 7.69 (6-34), and Oliveira’s study with FMA-motor of 53.35 ± 28.44 [25], [26]. This result may be because of the difference in stroke time; in particular, our study selected stroke patients in the early recovery stage; Husnul Mubarak’s study determined post-stroke patients with a stroke time of more than 3 weeks; Oliveira’s study only selected stroke patients over 6 months, that’s why stroke patients’ mobility is better than in the early recovery period.
The study results showed that the total FMA-motor score had a good correlation with the Barthel Index, with a correlation coefficient of r = 0.659, which is similar to the study of Oliveira et al. with a correlation coefficient r = 0.597 (P<0.05) [25]. Besides, this study’s total MI score strongly correlated with Barthel Index with r = 0.748. This result suggests that the level of physical activity measured based on FMA-motor and MI values affects the degree of independence of stroke patients in performing ADL. The high correlation coefficient in our study also shows that the FMA-motor and MI scores can be used as a reference to measure the degree of independence in post-stroke patients. However, in this study, we found that in those having paralysis of their dominant hand – the dominant hand group (right-handed patients have right-sided hemiplegia, left-handed patients have left-sided hemiplegia), FMA-motor had a higher correlation coefficient with BI (r=0.763) than that in the other group - the non-dominant hand group (left-handed patients have right-sided hemiplegia, right-handed patients have left-sided hemiplegia) (r=0.375). The results were similar when comparing the correlation between MI and BI in the dominant hand group (r=0.807) with the non-dominant hand group (r=0.537) (Table 5). This finding showed that the decline in movement and functional activity was more closely correlated in the dominant-hand group than in the non-dominant hand group. The cause may be the compensatory phenomenon when stroke patients use support from the healthy side in functional activities in daily activities. According to Olsen, the functional improvement of severely impaired patients after stroke can be attributed to compensatory strategies involving the non-paralytic limb [31]. It means no matter how patients perform tasks, the more important feature is their ability to perform functional activities to reintegrate into the community, regardless of whether compensatory strategies are used.
FMA-UE and FMA-LE were also significantly correlated with Barthel Index with correlation coefficients of 0.618 and 0.641, respectively. This result is similar to the study of Husnul Mubarak, with the correlation coefficient of FMA-UE and FMA-LE with Barthel Index of 0.739 and 0.820 (P<0.05) [26]. The subdomains of the Motricity Index, including MI-UE, MI-LE, and TCT, were also strongly correlated with the Barthel index with correlation coefficients of 0.596, 0.632, and 0.794, respectively. This result suggests that restoring the upper, lower extremity, and trunk motor function in post-stroke patients is essential in improving their independence. However, the study results showed that TCT correlated most strongly with BI. This finding suggests that trunk movement is a crucial factor in compensatory activities in post-stroke rehabilitation. According to studies, using the trunk is a compensatory strategy, which considerably correlates with the degree of functional impairment in post-stroke patients. During stroke recovery, the nervous system can maintain resilience by replacing lost elements of motor patterns, such as elbow extension and shoulder adduction, with new features, such as trunk movements, to achieve functional goals [32].
Our study showed that scales such as FMA-motor and MI strongly correlate with BI and can be a valuable tool to support the comprehensive assessment of activity and motor function according to the ICF model in post-stroke patients. According to Wade et al., there are some limitations when we measure individual functional abilities using only the ADL scale, so we should use more than one scale in the assessment [33]. Besides, our study also had some differences from other studies: (1) Our results showed that in the group of people with dominant-hand paralysis, the impairment in motor and activity function was more closely correlated than the other group, so the use of FMA and MI can assist in the assessment of motor recovery compared to using only the BI scale, especially in the non-dominant hand group. (2) Our study only included patients with cerebral infarction in the early recovery period and having Barthel under 60; this apparent characteristic can increase the accuracy of the scales. Many groups of participants with different characteristics, such as hemorrhage or infarction and mild or severe motor deficits, can confound the reliability of the measurement scales. In other words, evaluating similar subjects can improve the accuracy and statistical power in clinical trials using comparison and evaluation scales.
However, there are some limitations to this study. First of all, the study was conducted in multiple centers, so the reviewers’ assessment may not be completely homogeneous, which could compromise the objectivity of the evaluation.
However, according to the Rehabilitation Institute of Chicago, FMA only needs reviewers to read the manual; MI and BI do not need them to be trained, and these scales are also standardized and commonly used worldwide [17], [26], [34]. In addition, the evaluators in our study were instructed in using these scales through videos and tutorials. Several studies suggest that evaluators are trained or even only read a standard manual that provides detailed instructions on the process and tool of scale assessment can help enhances inter-reliability [35], [36]. Secondly, the FMA scale is a standard scale that evaluates many different factors in post-stroke patients, such as Motor function, Sensation, Balance, Range of motion of Joint, and Joint pain; however, our study only assessed the Motor functioning of the FMA, so future studies can generalize other criteria in FMA to evaluate patients more comprehensively. Finally, in the ICF model, many scales can be used to assess recoveries after stroke, such as spasticity (MAS), upper limb mobility (ARAT, WMFT, 9-HPT, BBT), motor and muscle strength (MMT), functional assessment scales (FIM, SIS, SF-36). Because our study only compares the correlation of the three scales (FMA, BI, MI), future studies should evaluate the correlation of more scales, supporting the evaluation of many clinical aspects of the patient.
Conclusion
The study results showed a strong correlation between activity function measured by BI and motor function assessed by FMA and MI in post-stroke hemiplegic patients due to cerebral infarction. Therefore, the more severe the mobility impairment, the greater the patient’s activity function (ADL) impairments. In addition, this study suggests scales such as FMA, MI, and BI, when used together, can have complementary effects, helping to assess the patient’s motor activity and general function more clearly and precisely.