1. INTRODUCTION
Pathologies related to immunological system disorders are getting more and more common. This may be the consequence of immunodeficiency or over-immune response. In case that the body’s resistance decreases, the body is susceptible to external pathogens such as viruses, bacteria, fungi [1]. On the contrary, if the immune response become super strong, in some cases against the body itself, it causes autoimmune diseases [2]. Interleukin-2 (IL-2) is secreted mainly by activated T-lymphocytes and involved in the cell-mediated immune response. IL-2 stimulates proliferation of T and B lymphocytes cells for immune response (3). The quantification of IL–2 in culture medium helps to evaluate the in vitro inhibitory effect of medicinal extracts on proliferation of peripheral blood mononuclear cells (PBMCs), a cellular fraction enriched in lymphocytes.
Traditionally, there are many medicinal herbs that people use as traditional medicine to treat diseases related to immune system abnormalities. The use of medicinal plants in traditional medicine brings certain benefits to patients such as significant reduction in side effects compared to chemical drugs. Many studies have evaluated the effects of medicinal herbs in supporting the treatment of treatment of immune-related diseases [4-6]. Many plant parts have been widely used in the treatment of immune diseases in Vietnam, yet just few of them are known about their mechanisms of action. The objective of this project is to investigate the antioxidant activity and the in vitro immunomodulation potential of medicinal plant extracts against stimulated PBMCs.
2. MATERIALS AND METHODS
Leucosep® tubes were from Greiner Bio-One. RPMI – 1640 media, FBS serum (fetal bovine serum), penicillin/streptomycin antibiotic, phytohaemaglutinin – M were obtained from Gibco. Trypan blue solution and phosphate buffered saline (PBS) 1X were obtained from Himedia. Thiazolyl blue tetrazolium bromide (MTT) and DPPH (2,2-diphenyl-1-picrylhydrazyl) reagent were obtained from Sigma - Aldrich. The human Interleukin-2 ELISA kit was obtained from Invitrogen. 96% ethanol was prepared for medicinal plant extraction. All reagent chemicals were under sterile conditions.
The parts of thirteen medicinal plants that are commonly used in the treatment of diseases in Viet Nam which were collected in Vĩnh Cửu District, Dong Nai Province in this study (see Table 1). Medicinal plant material was identified at Botany Department of Lac Hong University. Subsequently, medicinal plant material was washed to remove dust and mechanical impurities, dried in the air and then extracted by percolation.
Dried medicinal materials are crushed and sifted through a 2 mm diameter sieve. Twenty grams of medicinal plant powder were extracted in 200 ml of 96% ethanol for 72 hours at room temperature. The ethanol extracts were filtered out of debris with filter paper and then concentrated with a rotary evaporator at 50 °C to remove organic solvents, then left on a water bath at 60 °C until all the water of hydration is removed (designated as crude extract). The yield of dried extracts from starting crude materials was from 13.8% to 46.2% (see Table 1).
Fractionation of crude ethanol extract was carried out with different organic solvents to obtain chloroform, ethyl acetate (EA) and water fractions. Dried medicinal plant extracts of different solvents and water fraction were weighed and stored at −20 °C until use.
Preliminary qualitative phytochemical analysis was carried out on extracts to determine the chemical composition including test for polyphenol, saponins, alkaloids, triterpenoids, flavonoids, etc. according to standard procedures.
Test for polyphenol: After heating the extract diluted with water in a water bath for 15 minutes and filtering the suspension, 3 drops of 0.5% FeCl3 were added to the filtrate. The reaction was positive in the presence of a dark green or dark blue precipitate of tannins.
Test for saponins: 5 ml of extracts was heated until a dried matter in a test tube and then 5 ml of 25% ethanol was added. After heating test tube for 5 minutes in a water bath, the suspension was filtered and 5 ml of water were added to the filtrate. After agitation for 10 seconds, the appearance of stable foam showed a positive reaction in the presence of saponins.
Test for alkaloids: 5 ml of extracts was heated until a dried matter in a test tube and then 5 ml of 1% HCl was added. After rotating for 30s, 1 ml of the suspension was subsequently reacted with 5 drops of Dragendorff’s reagent and Bouchardat s reagent. The reaction was positive in the presence of precipitation of alkaloids.
Test for triterpenoids (Liebermann-Burchard reaction): After 5 ml of the extracts were dried and then dissolved in 3 ml of chloroform, a few drops of concentrated acetic anhydride and sulfuric acid were added. The reaction was positive in the presence of a violet ring of triterpenoids.
Test for flavonoid: 5 ml of extracts was heated for 5 minutes through the test tube and then a few drops of concentrated hydrochloric acid and a few amount of magnesium powder was added, respectively. The color changing into red or pink indicated the sample containing flavonoid.
Test for reducing sugars: 5 ml of extracts was heated for 5 minutes through the test tube and then some drops of Fehling’s reagent. The reaction was positive in the presence of red precipitate of reducing sugars.
The blood sampling for the purposes of this study was approved by the Ethics Committee of University of Medicine and Pharmacy at Ho Chi Minh City. Whole blood sample processing was conducted at aseptic conditions within 4 hours after blood collection. Five milliliters of whole blood samples from 10 healthy volunteers (5 men and 5 women) were anticoagulated with lithium heparin. The peripheral blood mononuclear cells (PBMCs) were extracted from whole blood using a leucosep® tubes according to the manufacturer’s protocol with minor changes (7). In brief, 5 ml of heparin blood samples were slowly placed in a leucosep® tube 10 ml. After centrifuging the leucosep® tubes for 20 minutes at 1200 g at 20 °C without braking, the plasma fraction was removed, PBMCs were collected and then washed twice with 10 ml of PBS with centrifugation at 200 g in 10 minutes at 20 °C. The cell pellet was resuspended in 250 μL of culture medium containing RPMI-1640, 10% FBS and 1% penicillin/streptomycin. PBMCs were stained with trypan blue and cell viability was evaluated using hemocytometer (viable cells over 95%). Then, cell density was corrected to 1×106 cells/ml using cell medium before conducting the experiment.
To investigate the inhibitory effect of extracts on in vitro human PBMCs proliferation, the crude extracts and fractions were dissolved in dimethyl sulfoxide (DMSO) and then diluted with culture medium to achieve concentrations of 0.1; 1; 10; 50; 100; 200 μg/ml applied on cell cultures.
For the assay, 100 μl of PBMCs suspension and 50 μl of culture medium with 10% phytohaematoglutinin (PHA) were added in 96-well plate to achieve a final concentration of 1×106 cells/well. For a sample test, cells were cultured with 50 μl of extracts at different concentrations. For a negative control, cells were cultured with 100 μl of culture medium with 10% PHA. For a sample blank, 50 μl of culture medium without PBMCs was added to 50 μl of extracts. For a blank, only 100 μl of culture medium with 10% PHA was plated in well of 96-well plate.
At 48h of incubation of coated wells at 37 °C, 5% CO2 and 90% humidity, 10 μl of 5 mg/ml MTT solution was added to each well. Formazan crystals were formed after 4 hours of incubation and then dissolved with 200 μl of DMSO/NH3 solution. The optical density (OD) and the reference wavelength were read in triplicate on a multi well scanning spectrophotometer (ELISA reader) at 555 nm and 690 nm, respectively. The concentrations (μg/ml) where 50% of PBMCs inhibited (IC50) or proliferative (EC50) were determined.
To evaluate the antioxidant activity of the extracts, the crude extracts and fractions were dissolved in methanol to achieve concentrations of 3.125; 6.25; 12.5; 25; 50; 100; 200; 400; 800 μg/ml for each extract.
The antioxidant activity of the crude extracts and fractions were determined using the DPPH free radical scavenging assay described by Teixeira J. et al. (8) with some minor changes. The 0.2 mM DPPH solutions as free radical factor and 2 μg/ml acid ascorbic (vitamin C) solutions as reference were prepared in methanol just before used.
For the assay, 100 μl of the extracts in concentrations from 3.125 - 800 μg/ml were added to 100 μl of 0.2 mM DPPH as the test samples, while the negative controls contained 100 μl of methanol and 100 μl of 0.2 mM DPPH in 96-well plate. The obtained mixture was vortexed, incubated for 30 min at room temperature and protected from light. The optical density was read at 515 nm. The blank control contained 200 μg/ml of methanol. The blank samples contained 100 μl of the extracts in concentrations and 100 μg/ml of methanol in 96-well flat as the test samples. Measurements were taken in triplicate. DPPH scavenging effect (DPPH %) was calculated by the following formula:
The results were reported as DPPH50 values as the effective concentrations (μg/ml) decrease 50% concentration of 0.1 mM DPPH in the assay.
To investigate the inhibitory effect of extracts on in vitro IL-2 production by PBMCs, the plant extracts that inhibit the in vitro PBMCs proliferation were diluted in culture medium at the concentrations of IC25, IC50, IC75 μg/ml, while the plant extracts that stimulate the in vitro PBMCs proliferation were diluted in culture medium at the concentration where PBMC proliferation was strongest.
For the assay, 100 μl of PBMC suspension and 50 μl of culture medium with 10% phytohaematoglutinin (PHA) were added in 96-well plate to achieve a final concentration of 1×106 cells/well. For a sample test, cells were cultured with 50 μl of extracts at different concentrations. For a negative control, cells were cultured in 100 μl of culture medium with 10% PHA. At 48 h of incubation of coated wells at 37 °C, 5% CO2 and 90% humidity, the medium in each well was collected and centrifuged at 1500 rpm for 5 minutes. The supernatant was collected for IL-2 measurement or stored at -20°C until assay. IL- 2 secretion by PBMCs in the cell culture medium was evaluated by immunoassay (IL-2 Human ELISA Kit, Invitrogen).
Data were processed and analyzed with GraphPad Prism Software Version 10.00 and Microsoft Excel 2010. The results were expressed as mean ± SD (standard deviation). The mean values of all the groups were compared by independent t-test. Statistical analysis was considered significant if p-value was <0.05.
3. RESULTS
Based on the results of the MTT assay, the effect of crude ethanol extraction of 13 medicinal plants on in vitro PBMC proliferation was initially divided into 3 groups: stimulating-group (2 medicinal plants), inhibiting-group (6 plants) and no effect-group (5 plants). The results of the proliferative activities of fractions from stimulating-group and inhibiting-group on PBMCs cells were shown in Figure 1 and Table 2. The chloroform extract of Wedelia chinesis showed the strongest inhibitory activity with an IC50 concentration 16.1 μg/ml, while the chloroform extract of Glycyrrhiza glabra showed the strongest stimulant activity with an EC50 concentration 10 μg/ml.
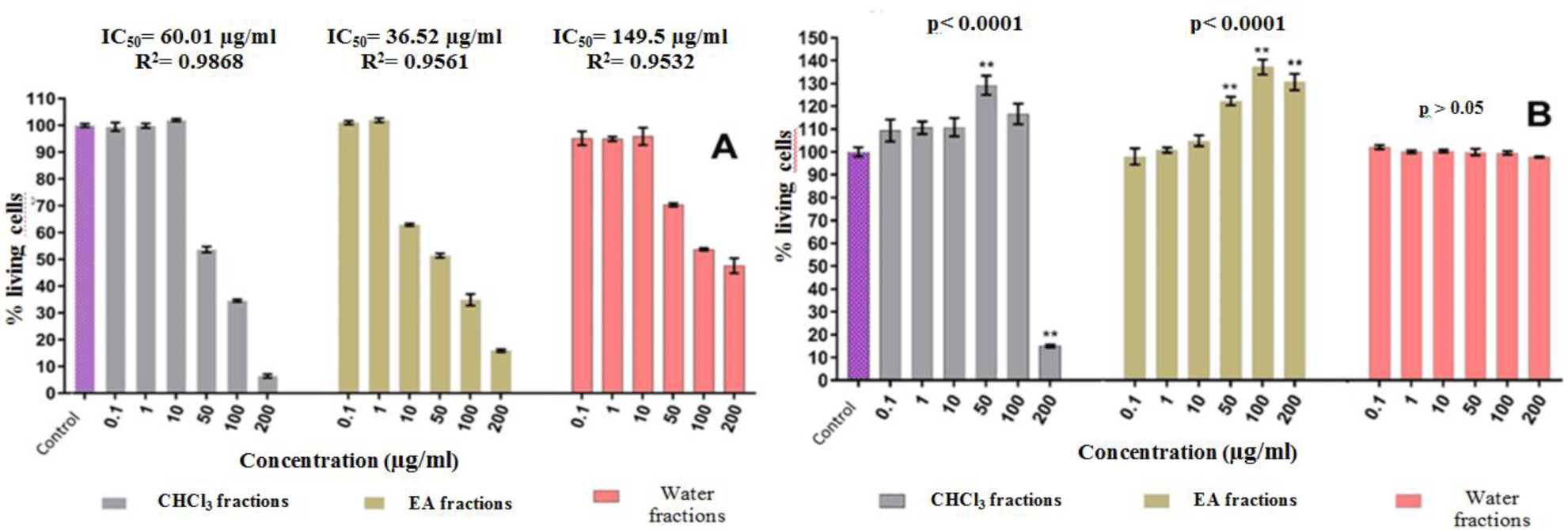
Preliminary qualitative phytochemical tests showed that the crude ethanol extracts and fractions from PBMC inhibitory medicinal plants group tested positive mainly for tritepenoids, flavonoids and total phenolic, while stimulant medicinal plants group contains primarily saponins and reducing sugars (see Table 3 and Table 4-A&B).
PBMC stimulating medicinal plants group have lower antioxidant activity than inhibitory PBMCs group. Most chloroform fractions have DPPH radical scavenging potential better than other fractions at different concentrations (μg/ml). The chloroform extract of Piper betle showed the strongest DPPH capture capacity with DPPH50 concentration 1.94 μg/ml and 2.1 times stronger than vitamin C (reference) (see Table 5).
The inhibitory extracts reduced the amount of IL-2, while the stimulant extracts had no effect on IL-2 secretion compared to the control (see Table 6).
4. DISCUSSION
The MTT test is used to measure the proportion of living cells that are active, while it is not possible to measure living cells that are inactive. Other methods may be used to confirm cell proliferation such as the cell cycle or the BrdU assay [9]. This study screened PBMCs proliferation using MTT assay and found 2 medicinal plants (Astragalus membranaceus and Glycyrrhiza glabra) stimulating PBMCs proliferation, 6 medicinal plants (Schefflera heptaphylla, Cassia alata, Piper betle, Lonicera japonica, Wedelia chinensis, Caesalpinia sappan) inhibiting PBMCs proliferation.
The compounds with antioxidant activity in pharmaceuticals include phenolic group, flavonoid, carotenoid, triterpenoid [10]. In this study, preliminary qualitative phytochemical test of the crude extracts and fractions showed that the medicinal extracts with inhibitory activity of proliferation of PBMCs contained phenolic, triterpenoid and flavonoid compounds. Moreover, the medicinal plants that stimulated proliferation of PBMCs (Astragalus membranaceus, Glycyrrhiza glabra L) had lower antioxidant activity than the medicinal plant group that inhibited proliferation of PBMCs. Most of chloroform fractions of the medicinal group have DPPH free radical scavenging activity higher than other fractions, in which the chloroform fraction of Piper betle showed a strong antioxidant activity with an DPPH50 of 1.94 μg/ml, as 2.1 times higher than that of vitamin C (reference). In comparison with the results of phytochemical analysis, the chloroform fractions are rich in triterpenoids (Piper betle, Schefflera heptaphylla, Lonicera japonica, Wedelia chinensis, Caesalpinia sappan), flavonoids (Wedelia chinensis, Caesalpinia sappan) and phenolic compounds (Piper betle, Schefflera heptaphylla, Lonicera japonica, Wedelia chinensis, Caesalpinia sappan). These chemical compounds could be able to capture DPPH free radicals.
In addition, triterpenoids have strong anti-inflammatory antiviral, antimicrobial, and immunosuppressive activities, so medicinal plants containing these compounds are commonly used empirically to treat diseases related to immune system abnormalities [11]. In our study, there is a relation between the antioxidant activity and anti-proliferative effects of the medicinal plant extracts on PBMCs, in agreement with the results of a previous study [12].
Interleukin-2 (IL-2) is secreted mainly by activated T-lymphocytes and involved in the cell-mediated immune response. IL-2 have effects on autoimmune diseases due to its influence in the differentiation of T helper cells, this directly influencing immune response processes. Therefore, IL-2 has an important role in the control and treatment of immune diseases [13]. Inhibition of IL-2 production is one of the mechanisms of immunosuppressive drugs [14]. The quantification of IL – 2 in culture medium helps to evaluate the effect of medicinal extracts on in vitro proliferation of peripheral blood mononuclear cells (PBMCs), a cellular fraction enriched in lymphocytes.
The preliminary qualitative phytochemical test of fractions showed that chloroform fractions with inhibitory activity of proliferation of PBMCs contained mainly triperpenoid, flavonoid and total phenolic compounds that have strong anti-inflammatory activities [11]. Therefore, these chloroform fractions were selected to examine their inhibitory effects on IL-2 secretion by PBMCs in this study. The results showed that inhibition of proliferation of PBMCs in culture media with high fractions may be associated with the inhibition of IL-2 production.
