1. INTRODUCTION
Parotitis is an inflammatory condition affecting the largest salivary gland in the body, occurring across all age groups and posing significant challenges in diagnosis and treatment [1–3]. This condition has diverse aetiologies, including infections, ductal obstruction, autoimmune disorders, drug-induced effects, and other factors such as trauma or radiation therapy [4–7]. Parotitis not only causes localized pain and discomfort but also affects salivary gland function and the patient’s quality-of-life [8–10].
Advances in imaging techniques such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), and sialendoscopy have enhanced the ability to identify underlying causes and assess glandular damage [11–14]. However, complex cases remain challenging to manage [1,15]. The lack of a standardised classification system and limited understanding of its pathogenesis further complicate diagnosis and treatment [16]. Despite ongoing improvements in medical and surgical therapies, recurrence and complications are still prevalent [1,10].
Therefore, the key question is: What are the most effective diagnostic and treatment strategies for different types of parotitis, and how can classification guide clinical approaches to improve patient outcomes? Given these challenges, it is essential to compile and update recent advancements in the diagnosis and treatment of parotitis. A comprehensive review can assist clinicians in making optimal treatment decisions and ultimately improve patient’s quality-of-life.
2. METHOD
This study is a literature review conducted by searching the PubMed and Google Scholar databases to identify studies related to parotitis. The search covered articles published in English on human subjects and was performed up to January 2025.
We conducted a literature search on PubMed and Google Scholar to collect studies related to parotitis. The search process utilized two main MeSH terms, “Parotitis” and “Sialadenitis”, combined with relevant topics such as “diagnosis,” “therapy,” “classification,” and “aetiology.”
Additionally, based on McAnally’s [17] classification, we expanded the search scope using specific keywords, including “parotitis,” “acute parotitis,” “bacterial parotitis,” “viral parotitis,” “chronic/recurrent sialadenitis,” “juvenile recurrent parotitis,” “adult recurrent parotitis,” “salivary duct obstruction,” “salivary duct stenosis,” “sialendoscopy,” “sialoendoscopy,” and “salivary endoscopy.”
Furthermore, we conducted supplementary searches by reviewing the reference lists of selected studies to ensure the broadest possible coverage.
Studies were included based on their research objectives on diagnosis, classification, treatment strategies, and clinical outcomes in parotitis patients. Eligible study types comprised systematic reviews, meta-analyses, randomized controlled trials, cohort studies, case-control studies, and case reports. Additionally, editorials and letters to the editor were considered if they provided relevant insights into these areas. Studies were excluded if they focused on animal models or non-human subjects or were not published in English.
Data extraction was conducted systematically to ensure the reliability and reproducibility of the synthesized evidence. One reviewer performed the initial search across selected databases, followed by independent screening of study abstracts by two reviewers to assess relevance. In cases of disagreement, the full text was reviewed, and a final decision was made through discussion.
Data extraction focused on diagnostic criteria, classification systems, and treatment strategies for parotitis. Relevant information on clinical presentation, imaging findings, and therapeutic approaches was synthesized to provide a comprehensive overview of the condition.
3. ANATOMY OF THE PAROTID GLAND
The parotid gland is the largest salivary gland, classified as one of the major salivary glands along with the submandibular and sublingual glands. Located in the lateral cervical region, it has a prism-like shape consisting of a main body and two extensions, closely associated with the masseter muscle, the facial nerve, the external carotid artery, and the retromandibular vein. The Stensen’s duct originates from the anterior part of the gland, passes through the masseter muscle, and opens into the oral mucosa opposite the second upper molar. The parotid gland is divided into a superficial and deep lobe by the facial nerve, a crucial anatomical feature in surgical procedures to prevent nerve damage [7,11,18].
At the microscopic level, the gland consists of parenchyma and stroma. The functional unit, the salivon, includes acini, intercalated ducts, and striated ducts. The acini are primarily composed of serous cells, which secrete enzymes such as amylase, lysozyme, and lactoferrin through a merocrine secretion mechanism [19].
4. AETIOLOGY
Parotitis is a disorder with various aetiologies, which can be classified into major groups including infectious, non-infectious inflammatory, mechanical factors, and other causes.
Parotitis due to infection is commonly caused by bacteria or viruses. The most frequent bacterial pathogens include Staphylococcus aureus, Streptococcus viridans, and anaerobic bacteria from the oral microbiota, while in neonates, group B Streptococcus is also a notable cause. Viral parotitis is most commonly associated with Paramyxovirus (mumps), along with other viruses such as Coxsackievirus, Cytomegalovirus, and Influenzavirus. Mycobacterium tuberculosis can also lead to parotitis, particularly in immunocompromised patients [7,19–22].
Autoimmune diseases such as Sjögren’s syndrome, systemic lupus erythematosus, and rheumatoid arthritis are common causes of chronic parotitis. These disorders often result in prolonged glandular damage, leading to impaired salivary secretion, xerostomia, pain, and persistent glandular swelling. Additionally, although rare, sarcoidosis and Heerfordt’s syndrome can also cause parotitis [7,15,19].
Mechanical-factor-induced parotitis is often associated with salivary duct obstruction. Parotid gland stones are a common cause, blocking salivary flow and leading to inflammation. Additionally, congenital abnormalities or Stensen’s duct stenosis can also result in recurrent parotitis [7,21,23].
Dehydration, malnutrition, and certain medications such as anticholinergics, antihistamines, and antidepressants can reduce salivary secretion, leading to fluid stasis and an increased risk of parotitis. Additionally, iodide toxicity and other chemicals can also affect gland function, causing salivary secretion disorders [5,24].
5. DIAGNOSTIC APPROACH TO PAROTITIS
The diagnosis of parotitis is primarily based on medical history and clinical examination to differentiate common causes such as infections, autoimmune diseases, or ductal obstruction. The initial assessment plays a crucial role in guiding appropriate diagnostic and treatment approaches (Fig. 1).
In bacterial parotitis, especially when purulent discharge is present from Stensen’s duct, Gram staining, bacterial culture, and antibiotic susceptibility testing should be performed to identify the causative pathogen and select appropriate antibiotics. Laboratory markers such as serum amylase, C-reactive protein (CRP), and erythrocyte sedimentation rate (ESR) may be elevated in parotitis but are not specific diagnostic indicators [7].
Imaging techniques play a crucial role in evaluating cases of parotitis with atypical presentations or suspected complex aetiologies. The most commonly used imaging modalities include:
Sialography is still widely used for diagnosing salivary duct obstruction, providing detailed visualization of the ductal system. This technique is valuable for assessing salivary stones, ductal stenosis, and anatomical abnormalities, aiding in effective treatment planning [25, 26].
Salivary gland ultrasound serves as the first-line imaging method, useful for detecting salivary stones, abscesses, differentiating solid from cystic lesions, and identifying hypoechoic areas caused by ductal dilation [11].
CT Imaging and the Role of CT Sialography: CT serves as an essential diagnostic tool for identifying salivary gland calcifications, particularly in cases of parotitis associated with sialolithiasis. Expanding on the diagnostic capabilities of standard CT, CT sialography combines advanced imaging techniques with contrast-enhanced visualization of the salivary ductal system. This approach offers superior spatial resolution and enables three-dimensional (3D) reconstruction, facilitating the accurate detection of small salivary stones, ductal narrowing, and structural anomalies that might not be apparent with conventional imaging. CT sialography is particularly advantageous for evaluating complex or deep-seated lesions, providing detailed anatomical insights that enhance clinical decision-making and support precise treatment planning, especially in surgical contexts [26,27].
MRI offers detailed information on soft tissue structures, helping differentiate chronic parotitis from malignant lesions. It also assists in diagnosing human immunodeficiency virus (HIV)-related parotitis by identifying characteristic cystic changes.
Sialendoscopy is effectively used for diagnosing and managing recurrent chronic parotitis. This minimally invasive technique allows direct visualization of the salivary ductal system, making it highly effective in identifying and treating salivary stones, ductal dilatation, and ductal strictures. It not only facilitates accurate diagnosis but also enables therapeutic interventions such as stone removal, ductal dilation, and irrigation, significantly improving patient outcomes [28].
Although generally not required for diagnosing parotitis, biopsy may be utilized in specific cases to support the diagnostic process. In suspected infections, a sample from the tail of the parotid gland can be cultured to identify the causative pathogen. For sarcoidosis, tissue analysis reveals non-caseating granulomas. In cases of suspected lymphoma, examining the tissue helps detect lymphocytic infiltration and destruction of glandular follicles, thereby aiding in accurate diagnosis [29].
6. CLASSIFICATION
Although several classification systems for parotitis have been proposed, there is a lack of consensus among clinicians regarding their applicability. Most recent classifications tend to focus on recurrent chronic parotitis rather than addressing parotitis as a whole. Therefore, an aetiology-based classification is considered a simpler and more practical approach in clinical practice. Among the existing classification systems, McAnally’s classification [17] is highly regarded for its clarity, comprehensiveness, and ability to encompass the clinical aspects of this condition [19] (Table 1).
7. FROM DIAGNOSIS TO TREATMENT
Viral parotitis, particularly caused by the mumps virus, primarily affects children and adolescents, presenting with characteristic clinical symptoms. Approximately 75% of cases exhibit bilateral painful parotid gland swelling, while 25% have unilateral involvement [19]. The disease typically begins with swelling on one side before progressing to the other. Accompanying symptoms include fever, fatigue, loss of appetite, and increased pain during chewing or swallowing. A key distinguishing feature from bacterial parotitis is the absence of purulent discharge from Stensen’s duct, which aids in accurate diagnosis.
Serological testing: Detects IgM or IgG antibodies specific to the mumps virus.
Viral RNA detection: Uses polymerase chain reaction to identify the virus in saliva, urine, or blood samples. Other causes such as HIV or other viral infections that may lead to parotitis should be ruled out.
Imaging: In cases of suspected complications or diagnostic uncertainty, imaging studies play a crucial role in the evaluation process. Ultrasound is often the first-line modality, as it may reveal gland enlargement without evidence of abscess formation. When there is suspicion of malignancy or severe inflammation, more advanced imaging techniques such as MRI or CT are recommended to provide detailed anatomical information and assist in establishing an accurate diagnosis.
The treatment of viral parotitis primarily focuses on supportive care and symptom relief. Patients should rest and drink plenty of fluids to prevent dehydration. Pain and fever can be managed with analgesics and antipyretics such as paracetamol or ibuprofen, and warm compresses on the parotid gland area may help alleviate pain and swelling. In terms of nutrition, soft foods are recommended, while acidic or spicy foods should be avoided to reduce irritation of the salivary glands. To prevent the spread of infection, patients should be isolated, especially unvaccinated children, and adherence to the Measles - Mumps – Rubella vaccination schedule should be ensured. Additionally, rare complications such as orchitis, pancreatitis, or meningitis should be monitored, with prompt medical intervention if they occur [2,7,19,30,31].
Sialolithiasis-associated parotitis is more common in adults, particularly males, and is diagnosed based on medical history and characteristic clinical symptoms. One of the hallmark symptoms is painful swelling of the parotid gland, which typically worsens after eating and gradually subsides within a few hours as salivary secretion decreases. In cases of infection, patients may experience a sensation of tightness and notice pus mixed with saliva [7,19,23]. Clinical examination may reveal a firm mass along the Stensen’s duct (if the stone is located near the duct opening), with turbid or purulent secretions in infected cases.
Imaging plays a crucial role in diagnosis, among which ultrasound is useful for determining the location and size of the stone, particularly those larger than 2 mm. X-ray or CT scans are effective in detecting calcium-containing stones, which account for approximately 80% of cases. MRI or sialography is indicated for complex cases to provide a comprehensive evaluation of the ductal system and rule out other lesions.
The treatment of sialolithiasis may include medical management, minimally invasive procedures, and surgical intervention. Medical management involves stimulating salivary secretion using sour foods or sialogogues, administering antibiotics if an infection is present, and applying warm compresses to relieve pain and improve salivary flow [7,32,33]. Minimally invasive techniques such as sialendoscopy allow for the detection and removal of small stones, while ductal dilation can help expel smaller calculi [27,32,34,35]. In cases of large stones or recurrent inflammation causing significant damage, surgical intervention should be necessary, including transoral stone removal (for stones near the Stensen’s duct orifice) or parotidectomy [32,36].
Drinking plenty of water can help reduce the risk of stone formation [19]. Additionally, dietary adjustments, such as avoiding foods that cause dehydration or increase the viscosity of saliva, may be beneficial [36]. Sialolithiasis-associated parotitis generally has a good prognosis if detected and treated promptly. Minimally invasive techniques, such as sialendoscopy, are increasingly favoured due to their effectiveness and low complication rates [34].
Acute suppurative parotitis is commonly seen in elderly individuals, immunocompromised patients, or those with prolonged dehydration (postoperative or chronic illnesses). Typical symptoms include sudden onset of painful swelling in a unilateral parotid gland, with overlying skin appearing red, tense, and warm. Purulent discharge from Stensen’s duct may be observed upon gland compression. Systemic symptoms such as high fever, chills, and generalized fatigue may also be present [2,7,22].
Laboratory tests: Pus culture plays a pivotal role in the evaluation of parotitis, as it is essential for identifying the causative bacterial pathogen. The most commonly isolated organisms are S. aureus and anaerobic bacteria, particularly species of Prevotella and Porphyromonas, which are frequently associated with suppurative infections. In addition, a complete blood count often reveals an elevated white blood cell count, accompanied by increased levels of CRP and ESR, which are indicative of an acute inflammatory response [22].
Imaging should be performed in the assessment of suppurative parotitis, especially in cases with suspected complications. Contrast-enhanced CT is particularly valuable for detecting large abscesses or identifying the spread of infection to adjacent structures, which is critical in patients at risk of developing mediastinitis or deep soft tissue infections. Moreover, ultrasound serves as a useful, non-invasive imaging modality to identify abscesses or fluid collections within the parotid gland, aiding in both diagnosis and management planning [37,38] (Figs. 2 and 3).
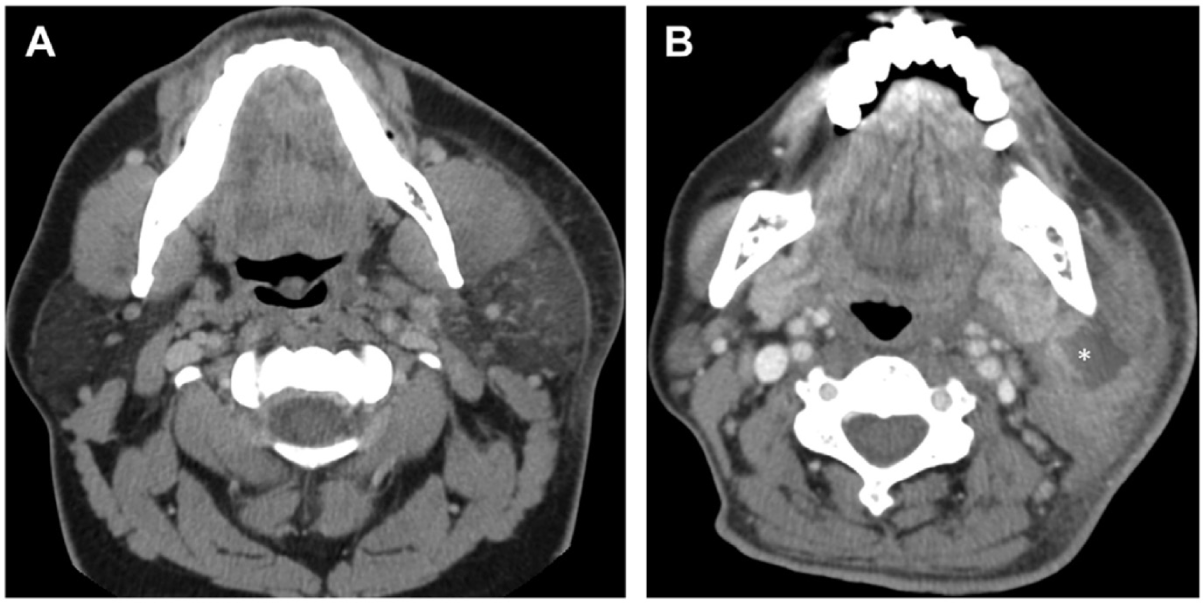
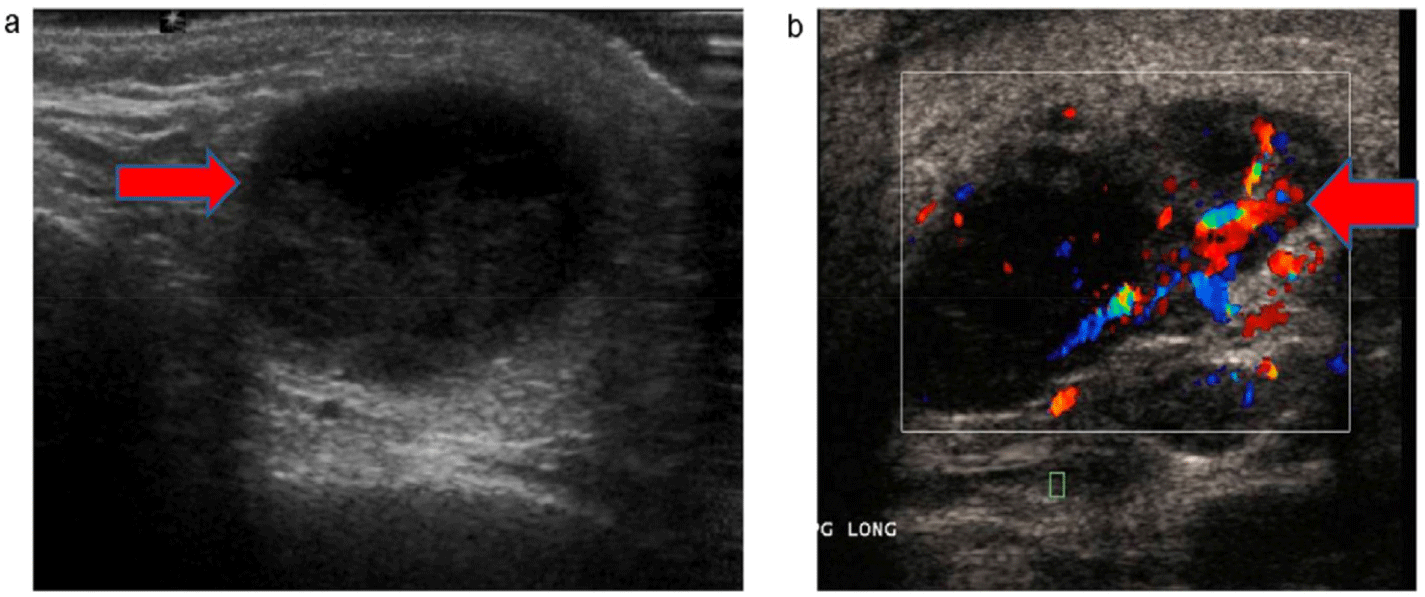
Empirical medical treatment for acute suppurative parotitis includes intravenous antibiotics for 7–10 days, with first-line options such as amoxicillin-clavulanate or ceftriaxone. In cases of suspected methicillin-resistant S. aureus (MRSA), vancomycin may be used. If anaerobic bacterial infection is suspected, clindamycin or metronidazole should be added to broaden antimicrobial coverage. Additionally, patients should receive analgesics and antipyretics such as paracetamol or NSAIDs, along with intravenous fluid therapy for severe dehydration to alleviate symptoms and support recovery [2,7,22].
In severe cases requiring further intervention, drainage of large abscesses should be performed, either by ultrasound- or CT-guided incision and drainage, ensuring no injury to the facial nerve. Irrigation of the parotid gland with antiseptic solution through Stensen’s duct can help remove pus and bacteria. Additionally, monitoring for signs of infection spread, such as cervical cellulitis or mediastinitis, is essential. Addressing risk factors like dehydration, poor oral hygiene, or underlying conditions (diabetes, Sjögren’s syndrome) is also crucial. Surgery is only indicated in cases of severe inflammation, abscesses unresponsive to treatment, or significant glandular damage, typically involving partial or total parotidectomy [2,15,27].
Acute suppurative parotitis is a serious infection that requires timely and aggressive treatment to prevent complications such as deep soft tissue infection, glandular necrosis, or systemic spread of infection. With appropriate antibiotic therapy and proper drainage, most patients have a good prognosis and achieve full recovery.
Chronic or recurrent parotitis is characterized by recurrent episodes of swelling and pain that persist for years, often unrelated to diet. Patients may experience unilateral or bilateral gland enlargement, typically mild and non-tender between flare-ups, along with reduced salivary secretion and persistent dry mouth. Upon gland compression, a greyish to slightly white precipitate may be expressed. Baurmash noted that while this substance is often mistaken for pus, it is not an indication of infection but rather a precipitate resulting from chronic inflammatory processes in the parotid gland [36]. During acute exacerbations, the gland becomes tense, painful, and may discharge pus through Stensen’s duct. In such cases, other causes such as Sjögren’s syndrome, bacterial parotitis, or malignant tumours should be ruled out [15,27,33,36].
Imaging: Sialography is an effective diagnostic method that provides detailed imaging of ductal dilation or obstruction within the salivary gland system. This technique can identify characteristic dilation patterns, such as punctate sialectasis, globular sialectasis, or a sausage-like appearance [36]. Additionally, modern imaging modalities such as CT and MRI are also used to detect chronic lesions and rule out other causes, such as glandular tumours or autoimmune diseases. The combination of these imaging techniques allows for a comprehensive and accurate assessment of salivary gland abnormalities (Fig. 4) [39] .
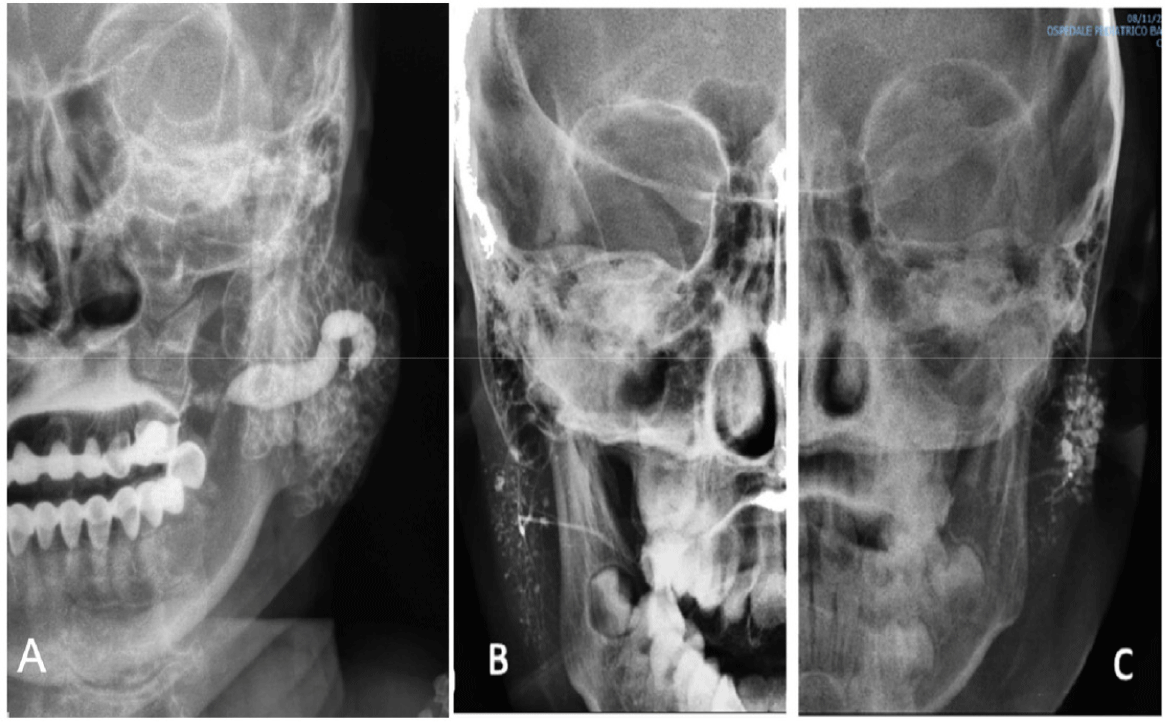
Ductal lavage is an essential step in the treatment of chronic recurrent parotitis, helping to restore salivary flow and reduce obstruction. The most commonly used technique involves irrigating the duct with saline solution for cleansing, combined with lacrimal duct probes to dilate narrowed ducts and improve salivary duct function [36]. A more advanced approach is sialendoscopy, which utilizes a salivary endoscope to directly visualize the ductal system, replacing lacrimal probes in removing precipitates and dilating stenotic areas. This method not only effectively cleanses the ducts and restores salivary flow but also enables precise diagnosis and therapeutic intervention during the procedure [28]. Due to its minimally invasive nature and high efficacy, sialendoscopy is increasingly being adopted for the management of recurrent parotitis caused by ductal obstruction or sialolithiasis [14,32,40].
Medical treatment for chronic recurrent parotitis includes anti-inflammatory and analgesic medications, with low-dose corticosteroids in cases of persistent inflammation. Salivary stimulation can be achieved using sour candies or cholinergic agents such as pilocarpine to enhance salivary flow. Antibiotics are only indicated during acute exacerbations when signs of infection are present to control inflammation promptly [15,27,36]. Supportive and preventive measures include maintaining adequate hydration to sustain salivary flow, improving oral hygiene, and avoiding risk factors such as dehydration, smoking, or alcohol consumption [7].
In the management of chronic recurrent parotitis, conservative treatment should be prioritized. Surgery is only considered when conservative measures fail. Surgical options include duct ligation, superficial parotidectomy, or total parotidectomy [36]. Surgery should be performed by an experienced surgeon to avoid facial nerve injury.
8. COMPLICATIONS
Although parotitis generally has a good prognosis, it can sometimes lead to complications, especially if not diagnosed and treated promptly. Potential complications include:
Parotid gland infection: Acute parotitis, if left untreated, can result in serious complications such as parotid abscess, cellulitis, and sepsis. An abscess may develop as a result of severe infection. This leads to the accumulation of pus within the gland, which causes pain and often necessitates drainage. If the infection spreads beyond the gland, it can cause cellulitis, characterized by swelling, pain, and overlying erythema. In rare cases, bacteria may enter the bloodstream, leading to sepsis, a life-threatening condition requiring intensive treatment [7].
Recurrent salivary stones: Chronic parotitis increases the risk of salivary stone formation, leading to recurrent ductal obstruction and inflammation [27].
Salivary gland dysfunction: Chronic inflammation may cause glandular tissue damage, resulting in reduced saliva production, dry mouth, difficulty swallowing, and an increased risk of dental caries.
Psychological impact: Persistent pain, swelling, and complications of parotitis can negatively affect a patient’s psychological well-being and quality-of-life.
Neurological complications: Prolonged inflammation or surgical intervention may lead to facial nerve injury, causing temporary or permanent facial paralysis. After parotidectomy, some patients may develop Frey’s syndrome, characterized by sweating and flushing in the preauricular region while eating [7,41–43].
Several factors increase the risk of complications in patients with parotitis. Immunosuppression, whether due to underlying diseases or immunosuppressive therapy, makes patients more susceptible to severe complications. Severe infections, particularly those caused by drug-resistant bacteria or those that spread extensively, also significantly heighten the risk. Additionally, delayed diagnosis and treatment can lead to disease progression and a higher likelihood of complications. To minimize these risks, timely diagnosis, appropriate treatment, and close monitoring for abnormal signs are essential.
This study has several limitations that should be acknowledged. First, as it is not a systematic review, the scope of the research may not comprehensively cover all literature on parotitis and does not apply quality assessment criteria. Additionally, the study only included articles published in English, which may introduce selection bias by excluding relevant studies in other languages. The reliance on data primarily from PubMed and Google Scholar may also result in the omission of relevant studies from other databases. Furthermore, there is currently no widely-recognized standardised classification system for parotitis. In this study, we refer to the classification by McAnally [17], as it is one of the few references addressing this topic. However, this system does not fully encompass all forms of parotitis and has not been widely standardised or validated, particularly in cases associated with autoimmune diseases such as Sjögren’s syndrome. Finally, treatment approaches for parotitis are highly diverse, especially for chronic recurrent parotitis, a condition that remains controversial in terms of management. Differences in study designs, diagnostic criteria, and outcome measures may affect the generalizability of the findings. Therefore, rather than delving into specific treatment techniques, this study focused on summarizing the most commonly used treatment strategies to provide practical clinical guidance.
9. CONCLUSION
Parotitis is a common condition that can occur at any age, presenting with diverse clinical symptoms that may be confused with other salivary gland disorders. Identifying the underlying cause of parotitis is the most crucial factor in treatment planning, allowing for appropriate interventions and reducing the risk of recurrence. Due to the variety of aetiologies, the rational application of ancillary diagnostic methods is essential for accurately determining the cause and assessing the extent of glandular damage. Parotitis is a significant condition that can lead to severe complications if not diagnosed and treated promptly. Therefore, specialists in Oral and Maxillofacial Surgery should enhance their awareness of this disease to ensure effective diagnosis and management, ultimately safeguarding patient’s overall health.









