1. INTRODUCTION
Puberty is a milestone in child and adolescent development for it is a transition stage characterized by many physical, and psychological changes which lead to rapid and substantial increase in body mass, stature, and thereafter the development of secondary sex characteristics. Body growth spurt occurs in this stage, thus pubertal onset is crucial to be correctly determined in daily clinical practice, especially if the child is undergoing abnormal pubertal maturation process i.e. precocious or delayed puberty.
Puberty status is regularly assessed by paediatricians in daily clinical practice. Medical tests such as invasive tests such as gonadotropin serum concentration or bone age radiography could be performed when abnormal puberty is suspected. Despite the high accuracy in results, these procedures are rarely applied in community research due to lack of human resources or adequate facilities. Self-assessment is an alternative, non-invasive method used to determine pubertal status; however, reported reliability and validity for this vary in literature [1-4]. The noted variations could be attributed to disparities in culture, socioeconomic status, education levels, and ethnics of the study population. In addition, coexisting conditions such as overweight and obesity can make assessment more difficult, especially among girls when assessing for breast assessment [5]. Although previous studies conducted on the Vietnamese population lacked measures of validation for self-assessment, this alternative still appeared in several works [6, 7]. In this research, we evaluated the validity of the puberty self-report questionnaire to provide evidence of a feasible and acceptable tool to accurately assess child and adolescent puberty status.
2. MATERIALS AND METHOD
Our cross-sectional study adopted the convenience sampling method. Sample size was calculated based on an equation for Kappa statistic estimation of two raters [8] using R function ‘N2.cohen.kappa’. Reference kappa coefficients were extracted from a study of Jaruratanasirikul et al. [2] and a minimum sample size of 40 boys and 60 girls was required. Approval for the study was obtained from Chamber of Education, District 10 and the administration of the selected schools. Study protocols and consent forms were sent out to the parents of children in selected classes among those schools.
Eligibility criterion of the study were as follows: students who were currently enrolled in selected schools were included in the sampling frame; students aging over 18 years old or having deformity that was not capable for demographic measurements were excluded. A total of 156 students (80 girls, 76 boys) in two elementary schools, two junior high schools and one high school were recruited from November 2017 to March 2019; convenient selection of 5 to 9 students of each gender for every grade was conducted by school administrators based on admission list.
Students were informed beforehand to wear light clothes (e.g. sports outfit) when physical measurements were taken. Body weight was measured by an electronic scale, standing stature was measured by a wall mounted stadiometer, weight and height were collected to generate body mass index (BMI). Anthropometric measurements were measured twice and mean values were used for statistical analysis.
Pubertal maturation — genitalia, pubic hair growth (for boys) and breast development (for girls) — was categorized in 5 stages according to Tanner (G1 to G5, PH1 to PH5, B1 to B5, respectively) [9]. Each child was asked to decide his or her maturation stage by checking a Tanner puberty stage self-report questionnaire, which was simply illustrated with drawings and short Vietnamese descriptions depicting 5 developing stages (Figure 1). Brief explanations could be delivered if the sexual and pubertal concepts were not familiar to the participants. After completion of the report, the child was examined by a same-sex paediatrician to determine his or her pubertal stage in a private room with the presence of a researcher. Age of menarche (in girls) and age of adult voice attainment (in boys) were recorded for sexual maturation interpretation using WHO criteria: prepubescent, pubescent, postpubescent [10].
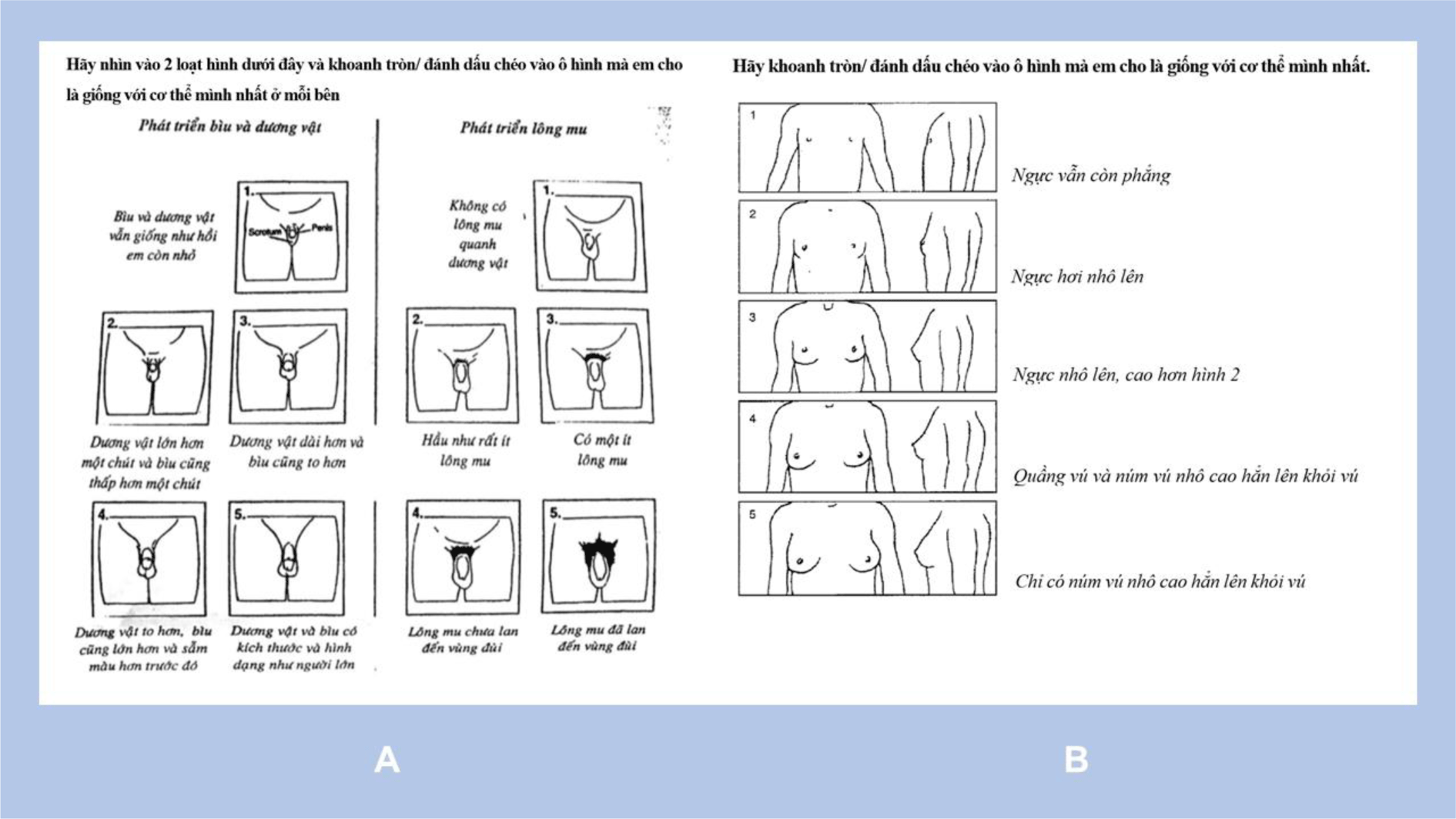
Data analyses were conducted using STATA 14.2 (Stata Corp.). Two sample t test and Fisher’s exact test were used to test the difference of anthropometric measurements among sexes, age groups, and pubertal stages. BMI-for-age z scores were calculated by zanthro command based on WHO Child Growth Standards [11]. Level of significance (α) was set at 0.05.
Students were divided into two age groups: from 6 to 11 years old and older than 11. Justification for the categories was that 11-year-old-and-below children in our sample shared similar characteristics as they were all elementary students, and it was less likely for them to experience puberty onset at that age [6].
Agreement between students’ self-assessment and paediatricians’ assessment was evaluated using kappa statistic. Quadratic weighted κ (Wκ) coefficient results were categorized as follows: less than 0.00 was poor agreement, 0.00 to 0.20 was slight agreement, 0.21 to 0.40 was fair agreement, 0.41 to 0.6 was moderate agreement, 0.61 to 0.80 was substantial agreement, more than 0.80 was almost perfect agreement. The concordance of two assessments measured by Kendall’s τ b was annotated as perfect concordance if Kendall’s τ b was equal to +1, perfect discordance if Kendall’s τ b was equal to -1, and no association if Kendall’s τ b was 0.
This study was approved by Pham Ngoc Thach University Scientific Committee (number of approval: 2305a/QD-TDHYKPNT). Written consent forms were collected from students and parents. Consent forms were presented to parents for signing and thereafter obtained before the start of data collection. The participants reserved the rights to withdraw from our study without any explanation and penalty.
3. RESULTS
Demographic characteristics of the sample are described in Table 1. In general, most of the characteristics were not significantly different between boys and girls, except for weight. Overweight and obese students accounted for 41.02% of all participants, and half of the cases were allocated in elementary schools.
Boys tended to overestimate their pubertal status while opposite pattern was observed in girls. Almost half of the boys determined their genital development higher than their current statuses; 20.27% thought that they were at higher stage in pubic hair development, and only 6.76% thought they were at lower stages. Of all girls, the proportion of underestimation was 33.75% and the proportion of overestimation was 17.5%. There were 8 boys (4 of them were older than 11) whose self-reported sexual maturation could not be determined because age of adult voice attainment came before appropriate genital development (G3).
Agreement analyses revealed that younger boys had limited awareness of their Tanner development stages (p values > 0.05 in all criteria) while their female peers showed better agreement and concordance with paediatricians’ evaluation (Wκ=0.04, p value < 0.05; Kendall’s τ b =0.44, p value < 0.001, respectively) (Table 2). Male adolescents had moderate to almost perfect agreement with paediatricians’ evaluation (genitalia: Wκ=0.59, Kendall’s τ b =0.51; pubic hair: Wκ=0.88, Kendall’s τ b =0.77, all p values < 0.001), whereas female adolescents fairly agreed with paediatricians’ result of their breast development (Wκ=0.38, Kendall’s τ b =0.39, all p values <0.05). Agreement on sexual maturation interpretation ranged from moderate to almost perfect, yet younger boys showed no agreement with experts’ categorization (Wκ=0.01, Kendall’s τ b =0.09, all p values > 0.05).
Subgroup analysis of pubertal self-report agreement among overweight and obese schoolgirls showed similar result to non-overweight girls. Wκ for breast development agreement in overweight/ obese student group was 0.79 (p <0.001) and the value of this coefficient in non-overweight group was 0.74 (p<0.001). In boys, pubic hair development agreement was also similar across two groups (Wκ=0.95, p<0.001 in overweight/ obese boys; Wκ=0.90, p<0.001 in non-overweight). Higher level of agreement in genital maturation was recorded in non-overweight schoolboys (Wκ = 0.58, p<0.001) in comparison with overweight/ obese group (Wκ = 0.41, p=0.004).
Of all the girls in our study, 50% of them declared to have had their first menstruation. Age at menarche ranged from 7.56 to 14.14 years old; median menarcheal age was 11.54 (95% CI: 10.6 - 12.76) years old. Median age of adult voice attainment in boys was 12.96 (95% CI: 11.7 - 13.50) years old.
4. DISCUSSION
This is the first study in Vietnam to assess the possibility of having children and adolescents report their own pubertal status using a Tanner stages paintings questionnaire. Moderate to high level of agreement and concordance were recorded in all three criteria for both sexes.
Elementary male students showed poorest agreement to paediatricians’ assessment results. This could be explained by the fact that children may find it hard to make comparison between their current body appearance and the paintings, especially the development of testicles in boys. Furthermore, primary school students who have not had sex education lessons in the mandatory education scheme may not understand the descriptions thoroughly even if researchers had briefly explained these. To counteract these outcomes several assistive tools could be provided to the child to make assessment easier. One study used a full length mirror for the child to look at his or her body to decide at which stage he or she was [3]. Another study spared a private space in the examining room for the child to undress and self-examine his sexual characteristics prior to Tanner stage decision [12]. In both of the aforementioned studies the genitalia assessment results were higher than our study as Wκ coefficients were 0.60 and 0.63, respectively. However, these two recommendations are quite unfeasible in community setting where the studies are carried out in remote areas with lacked privacy for the child to self-examine. Inaccurate puberty estimation in the self-report method among younger boys, however, yielded little impact in overall interpretation because boys aged from 6 to 11 years old were not likely to experience puberty onset, and delayed puberty was more prevalent in boys than precocious puberty. High level of agreement in breast stages in girls and almost perfect agreement in pubic hair distribution assessment in boys in our study were consistent to other previous findings [1-3].
In terms of sexual maturation interpretation, self-report method showed values as moderate agreement was recorded in male adolescents and moderate to almost perfect agreement was recorded among girls of all ages. However, adult voice was a vague definition for students, especially younger ones, to understand. Misunderstanding may have led to uninterpretable results in our study.
Peterson et al. recommended an interview-based self-reported method to interpret children’s pubertal status, known as Pubertal Development Scale [13]. This method showed advantages as it required least exposure of the child to clinical examinations or sexual maturation photos, therefore, it would be more easily accepted by parents and students. However, this measure insisted a trained health professional to interview and make questions to clarify information when participants expressed confusing feelings about the sexual characteristics or made unsuitable answers.
Age of menarche in our study was a little later than other studies with similar population. Mean menarcheal age was 11.2 years old in a study of junior high school students [6] and median age of menarche was 10 in another study of primary students [14].
While the patterns in overweight and obesity prevalence were similar to other studies done in Vietnam [15, 16], our study found a higher overall prevalence with greater frequency among boys.. Furthermore, Hong et al., in their report, pointed out that there was an increasing trend in overweight and obese prevalence over time and that there were more overweight and obese adolescents in urban areas than rural areas. Our findings may be affected by the sampling area: selected schools were located within centre of Ho Chi Minh City which was a highly developed and “wealthier” area in comparison with others.
Overweight and obesity were concerns to the reliability of breast self-assessment among girls in literature [5]. Our study found no detectable difference in breast development agreement between overweight/ obese and non-overweight students, Wκ coefficient in overweight/ obese group was 0.79, which was higher than another finding in Thailand (Wκ = 0.41) [2].
The strength of our study is the wide age-range of participants which allowed us to investigate validity of puberty self-report questionnaire of different stages of development. The limitations included a small sample size which caused difficulty in result interpretation. Lack of representativeness makes it difficult to generalize our findings to whole population.
Conclusion
Our study provides evidence for the validity of using Tanner pubertal stages self-report questionnaire as a sufficient method to evaluate children and adolescents’ sexual development status. Although this method was inaccurate to evaluate younger boys’ sexual maturation, acceptable accuracy in sexual maturation estimation among younger girls and adolescents could serve as an effective screening tool in community context.







