1. INTRODUCTION
In recent years, there has been a remarkable increase in the medical uses of plant-derived products [1]. Approximate 80% of the world population relied on traditional medicine for their health care [2]. Hydnophytum is a genus of epiphytic myrmecophytes (ant plants) belonging to the Rubiaceae family. Many species of the genus Hydnophytum are native to Southeast Asia. Hydnophytum formicarum Jack. is one of the species of Hydnophytum, growing on Phu Quoc Island, South of Vietnam. The tuber of H. formicarum has traditionally been used for the treatment of rheumatism, arthritis, liver and intestinal diseases [3]. In Asia, some studies showed that extracts of this medicinal plant have anti-oxidative, anti-bacterial, cytotoxic and hypoglycemic effects [4, 5].
Our study has been performed to evaluate the botanical, pharmacognostic, phytochemical and DNA features of H. formicarum collected from Phu Quoc Island which could serve as criteria of identification and authentication.
2. MATERIALS AND METHODS
The fresh plants of H. formicarum with a uniform structure were collected from Phu Quoc, Kien Giang, Vietnam on July 2018 for the study on DNA barcodes and on February 2014 for the others. The voucher specimens were kept at the Department of Pharmacognosy, Faculty of Pharmacy, University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam.
The following chemicals and reagents were used: absolute ethanol, ether, ferric chloride, sodium hydroxide, lead acetate, sodium carbonate purchased from Chemsol Company, Vietnam. Folin - Ciocalteu reagent and isopropanol were obtained from Merck & Co., Inc, Germany; pyrogallol was purchased from Sigma-Aldrich Co., USA. CTAB buffer (CTAB 2%, Tris-HCl 100 mM pH 8.0, 20 mM EDTA pH 8.0, 1.4 M NaCl), β-mercaptoethanol, chloroform: isoamyl alcohol (24:1), Rnase and agarose enzymes was obtained from Bio Basic Inc., Canada; PCR Mix was provided by NEXproTM, Genes Labs Inc., Korea; GelRed dye, TAE 1X cushion, loading dye 6x, 1 kb plus ladder and TE pH 8.0 from Promega Co., USA.
The fresh plants were washed thoroughly with distilled water and air-dried according to the instructions of herbarium technique standards. The tubers were then cut and ground to powder stored in airtight bags for further uses.
The macroscopic features of stems, roots, leaves and tubers were described. For the anatomical study, cross sections of the fresh plant organs were prepared and stained with iodine green-carmine then washed with water. The sections were then observed through a microscope. The microscopic characteristics of the tuber powder were also observed. The photographs were taken via an optical microscope and a Canon digital camera.
All the investigated fresh leaves of the plant were frozen, dried and ground to a fine powder with liquid nitrogen prior to DNA extraction.
DNA was extracted from 100 mg of fresh leaf using the CTAB protocol described by Doyle and Doyle (1990) [6]. DNA was submitted to electrophoresis on 1% agarose gel containing 1 μl GelRed to check the integrity and purity. The electrophoresis result was photographed under UV light. The extracted DNA samples were then stored at -20°C for future use.
PCR amplification was performed in PCR GeneAmp PCR System 2700 thermocycler (Amplied Biosystems, Singapore) using PCR kit (NEXproTM Diagnostics). PCR was carried out using 50 μl of reaction mixture containing 10X e-Taq buffer, 10 mM dNTP, e-Taq DNA polymerase, primers rbcL and distilled water. The thermocycler was programmed for 35 cycles, each cycle included 5 mins at 95°C for initial strand separation, 30 secs at 95°C for denaturation, 30 secs for primer annealing at 60°C, 30 secs for primer elongation at 72°C followed by 5 mins of final primer extension at 72°C. PCR products were separated on 1% agarose gel by electrophoresis.
The PCR products were purified by Wizard SV Gel and PCR Clean-up System kit (Promega Co., USA), then sequenced by Sanger method [9] at Phu Sa Biochem Company in Vinh Long, Vietnam. The size and molecular weight of DNA bands were calculated by GelAnalyzer software [10]. The sequence result was stored in FASTA and analyzed by BioEdit 7.0.5 [11]. The sequences were compared to GenBank using the BLAST algorithm (Basic Local Alignment Research tool) [10]
The tuber powder was extracted with ether, ethanol and water respectively by maceration. The extracts were then concentrated. The extracts were then concentrated and used for preliminary phytochemical tests to identify alkaloids, saponins, flavonoids, coumarins, carbohydrates, tannins, steroids, triterpenoids, cardiac glycosides, fats, reducing compounds and amino acids by the improved Ciuley method [12].
Pharmacognostic parameters of tuber powder (moisture content, total ash value and acid insoluble ash of dried powder) were determined according to methods described in the 4th and 5th Vietnamese Pharmacopoeia (2014, 2017) [12, 13].
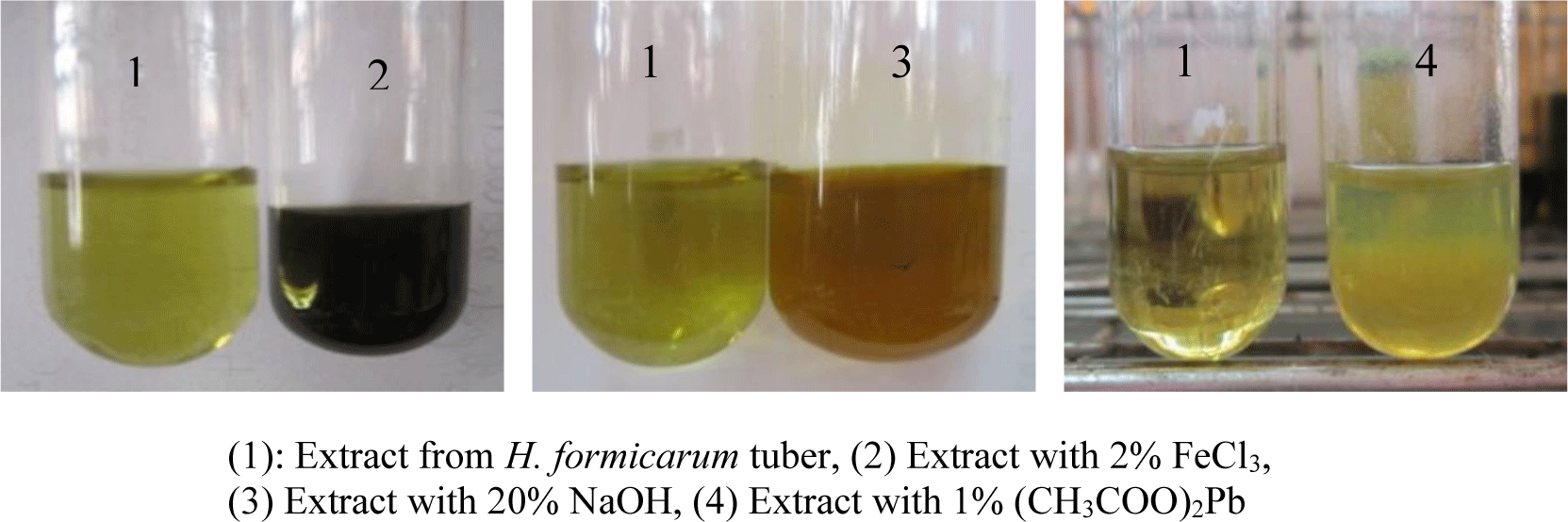
Five grams of tuber powder were extracted with 50 mL of 50% ethanol at 90°C for 2 hours. After filtration, this extract was used for histochemical tests to identify the presence of phenolic compounds with 2% ferric chloride, 20% sodium hydroxide and 1% lead acetate.
One gram of tuber powder was extracted with 50% ethanol solution until the filtrate did not change its color in reaction with ferric chloride solution. The filtrate was concentrated to obtain the crude extract. This crude extract was totally dissolved in water using a sonicator and then filtrated through a paper filter for total phenolics content determination.
The total phenolic content was determined by using the Folin-Ciocalteu assay [15]. The extract and the standard pyrogallol solutions at different concentrations (10-50 μg/ml) were taken to test tubes before 0.5 mL of Folin-Ciocalteu reagent (10%; w/v) was added. After 5 mins, 1.5 mL of sodium carbonate solution (29%; w/v) and 2.5 mL of distilled water were added to the mixtures and shaken. The mixtures were then incubated in 30 mins at room temperature under dark condition. After 5 mins of centrifugation at 5000 rpm to precipitate sodium carbonate crystals, the absorbances of solutions were measured at 760 nm with a UV-Visible spectrophotometer. The blank sample including Folin-Ciocalteu reagent, sodium carbonate solution and distilled water was carried out at the same time. The total phenolic content was expressed as mg of pyrogallol equivalents/g of dried tuber powder.
3. RESULTS
Macroscopic features: H. formicarum grows on trees in Phu Quoc Forest (Figure 1A). It is an epiphytic shrub with a tuber at its base also known as a caudex which is spineless and has two to four stems that reach about 60 cm long (Figure 1B). Slender stems, on which the leaves and flowers are born, grow out from the tip of the caudex. In contrast, leaves, which grow 4-15 cm long and 2-7 cm wide, are elliptical and have a leathery texture (Figure 1C and D). Swollen main stem (or caudex) is slightly bumpy, brown, 15-20 cm across and has many holes on the surface which are linked by numerous tunnel made by ants inhabit there (Figure 1E and F).
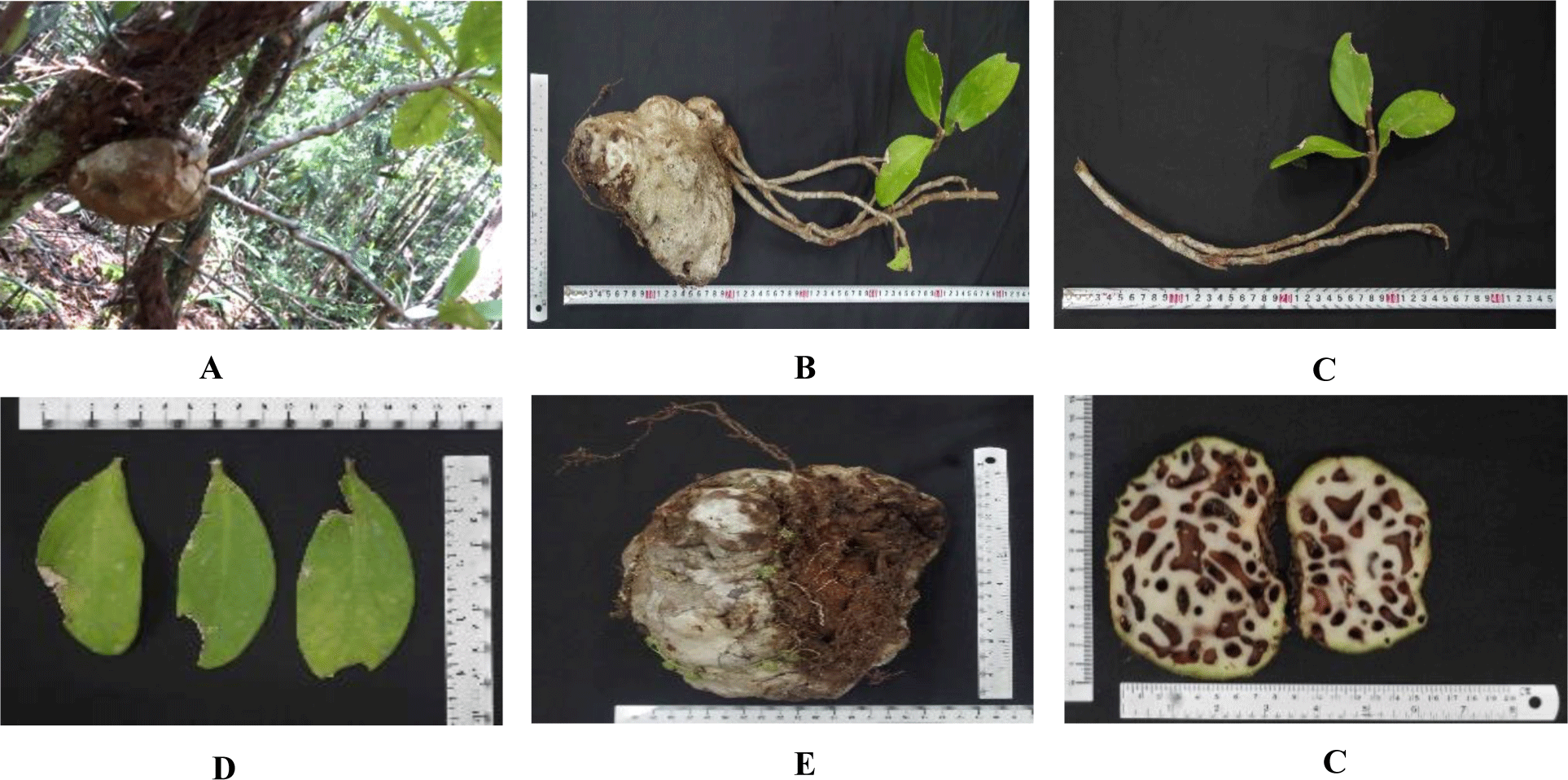
These characteristics have been described by Vo (1997). Because we could not collect the flowers, fruits and seeds of H. formicarum from Phu Quoc Forest, it was difficult to obtain fully macroscopic features for accurate identification of this species. Therefore, we added the study on DNA barcodes of H. formicarum from Phu Quoc Forest in this work.
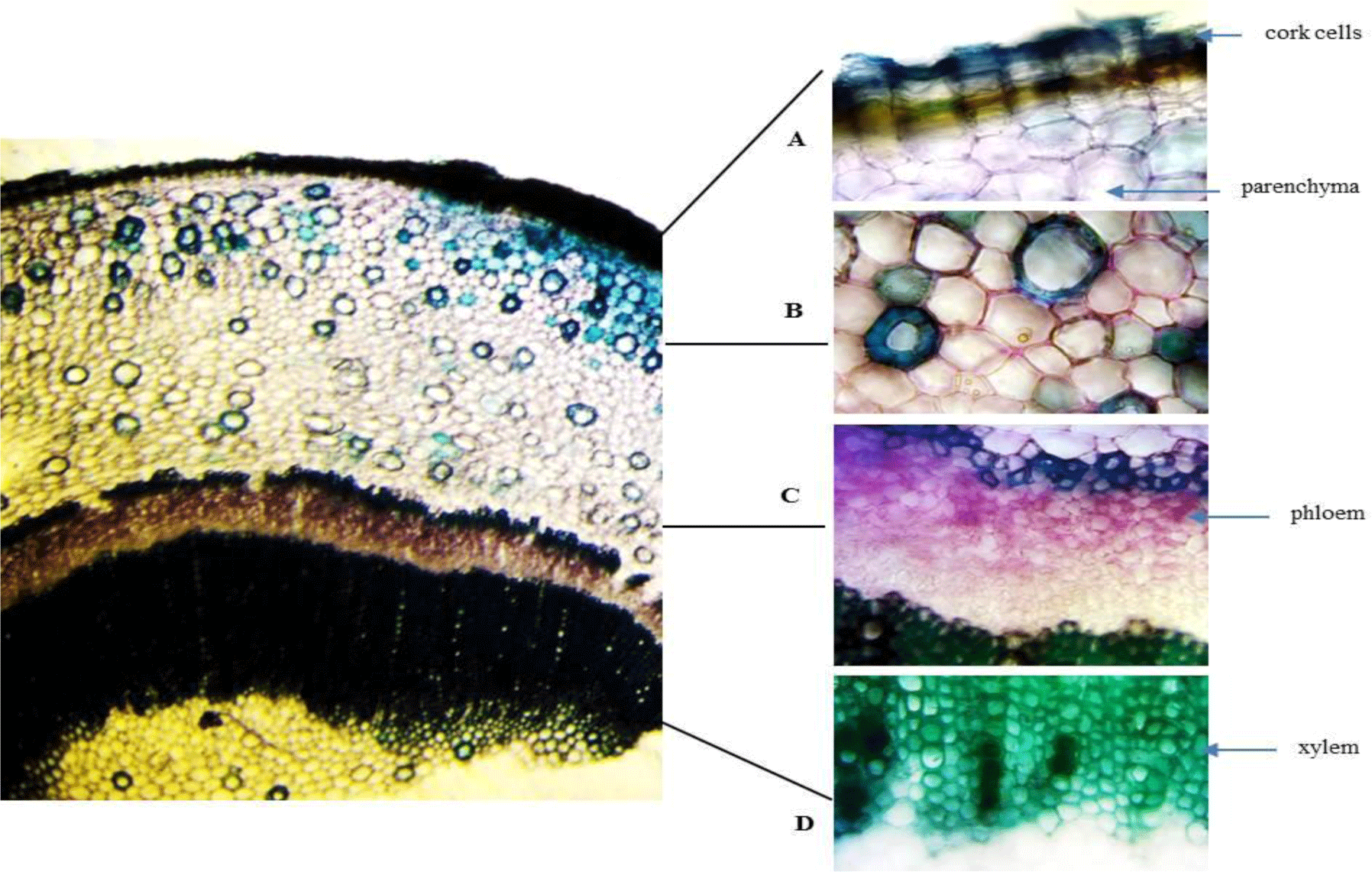
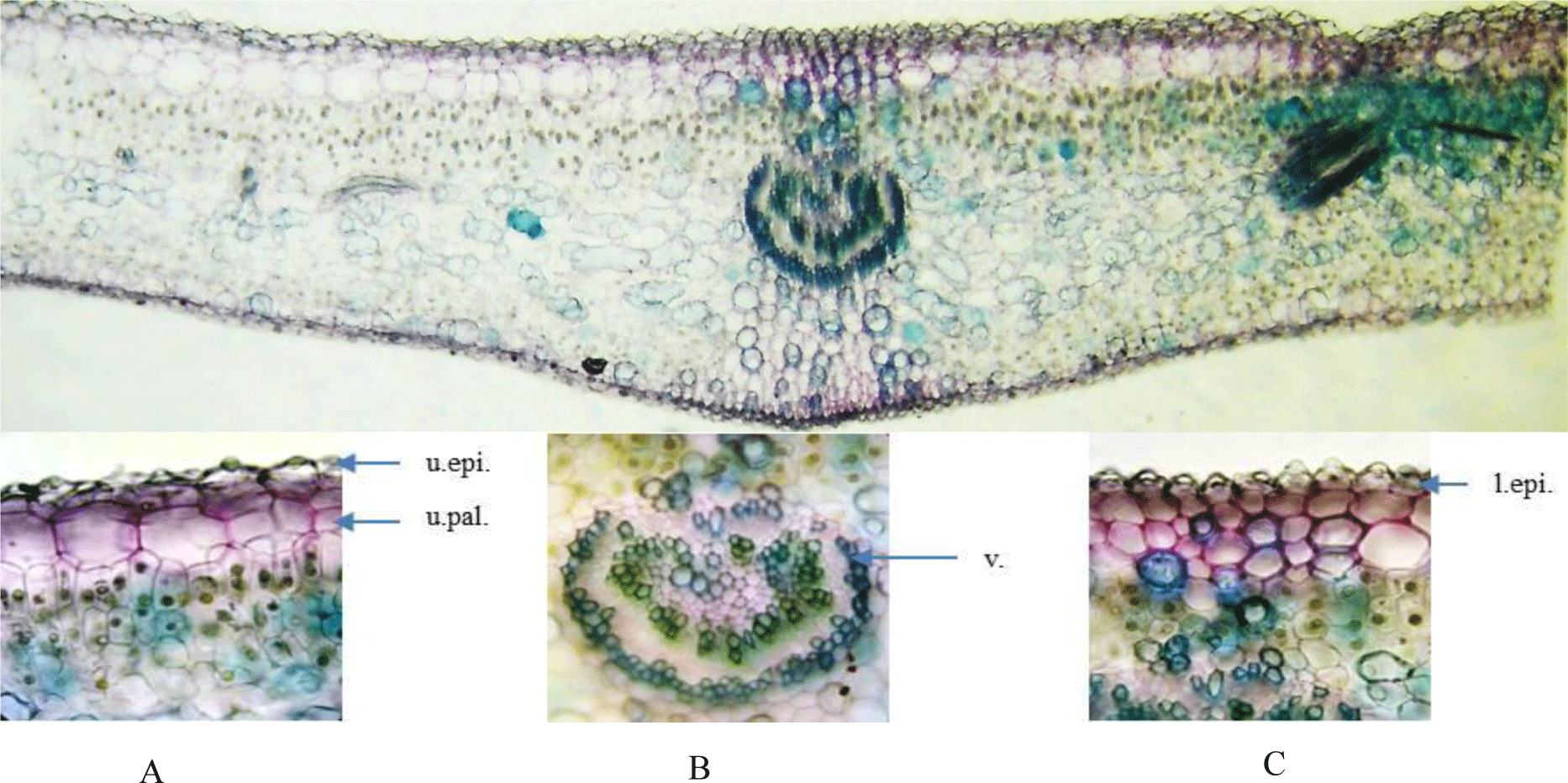
Stem: The stem has a circular outline. The outermost zone consists of radial bands of rectangular, tangentially elongated, thin-walled cork cells in 5-10 rows. The cortical zone comprises heterogeneous cells of parenchyma and big nests of sclerenchyma. The xylem and phloem are continuously arranged with a ring of fibers outside. The secondary phloem is composed of phloem fibers in small patches with thin-walled parenchyma in between. The vascular cylinder is composed of dense solid secondary xylem with small vessels. At the inner end of the secondary xylem, a ring of thickened and pitched cells is located. The parenchymatous pith presents in the center.
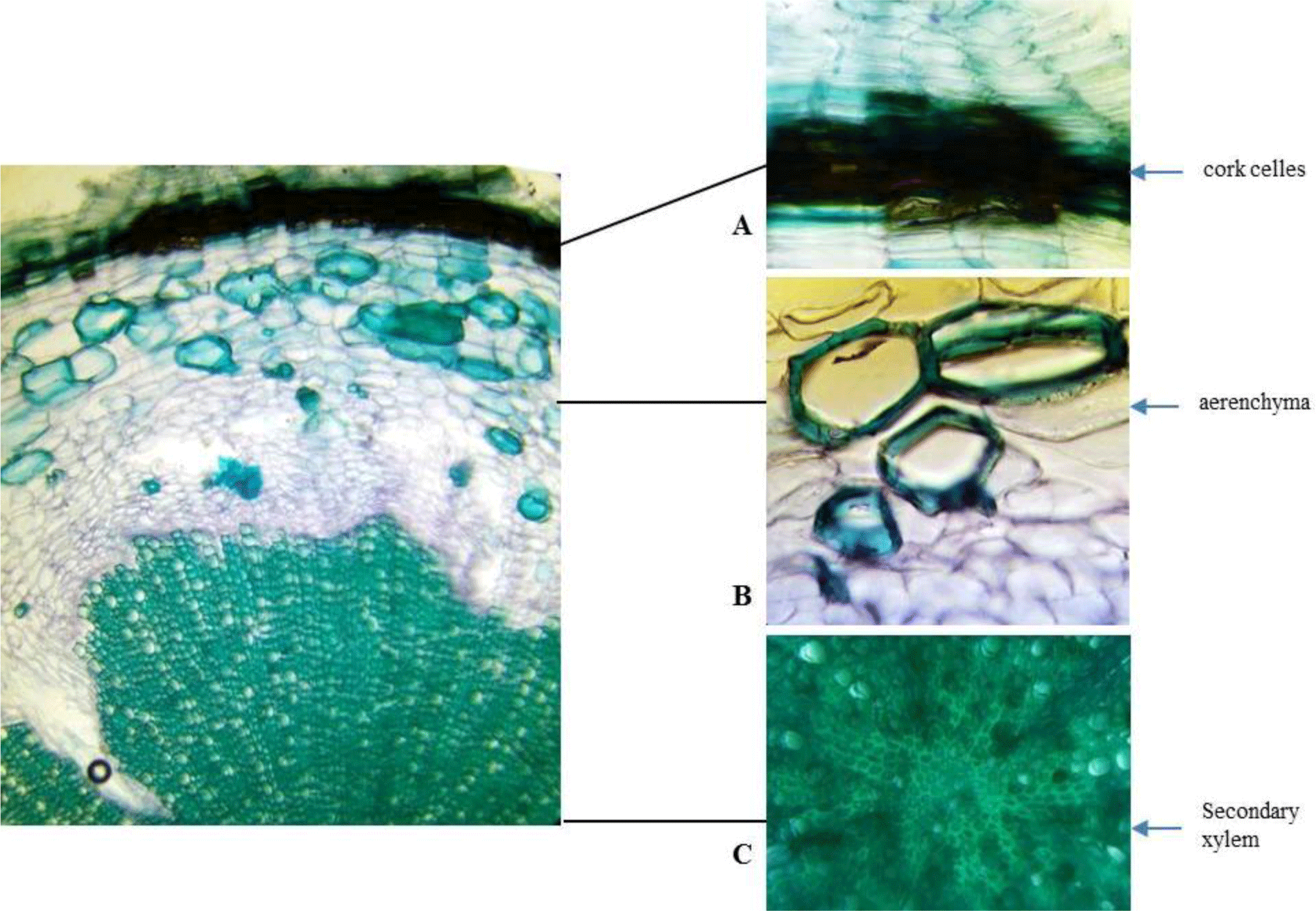
Root: Cross section of the root shows circular or oval shape in outline with no fissures. Cork consists of thin-walled, regularly arranged cork cells. Thin phelloderm is composed of tangentially elongated, slightly thickened cells. Inside phelloderm, there are many layers of parenchyma with a big nest of sclerenchyma and aerenchyma. Secondary xylem is narrow and parenchymatous. Secondary xylem is dominated by thickened, pitted fibers.
Leaf: Epidermis of the upper and lower side is covered with papillae, trichomes are absent. Cross section of leaf blade is bifacial with a single row of large hypodermis under the upper epidermis. Mesophyll cells are differentiated into palisade and spongy parenchyma. Palisade parenchyma below Vhypodermis consists of three vertically elongated cell layers. Spongy parenchyma lies below palisade parenchyma and is loosely arranged. Midrib has 34 layers of collenchyma under the epidermis. Vascular bundles are of the open type with a ring of sclereids outside.
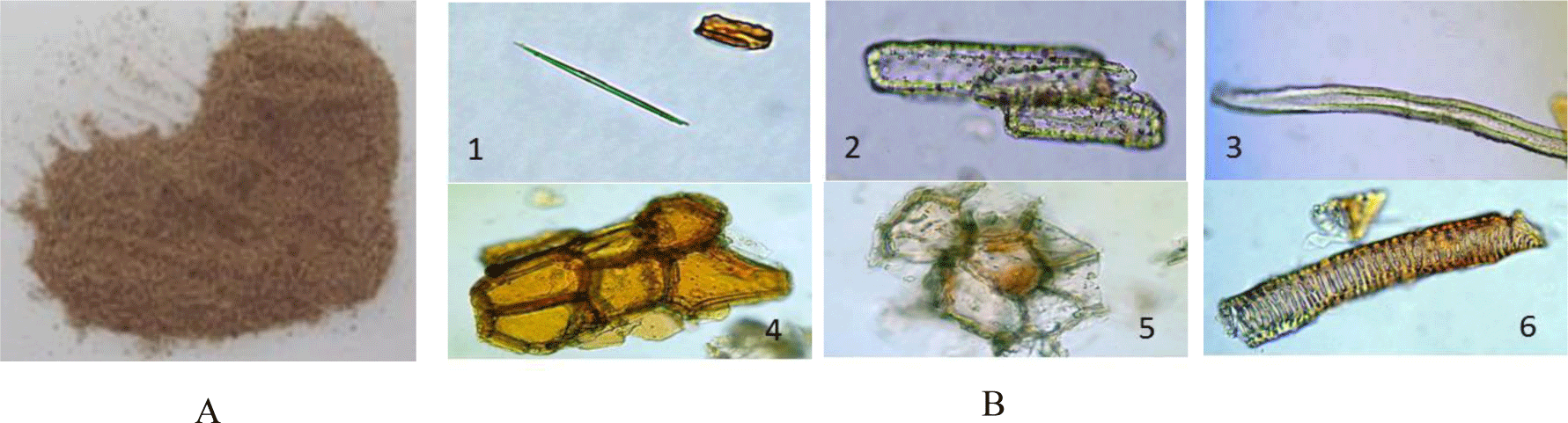
Powder characteristics: The dried tuber powder is yellowish, odorless and tasteless (Figure 5A). Under the microscope (Figure 5B) the powder shows acicular calcium oxalate crystals (1), groups of sclereids composed of elongated cells with thick wall (2), individual fibers (3), numerous fragments of cork with conspicuous granules (4), numerous fragments of thin-walled parenchyma (5) and elements of xylem (6).
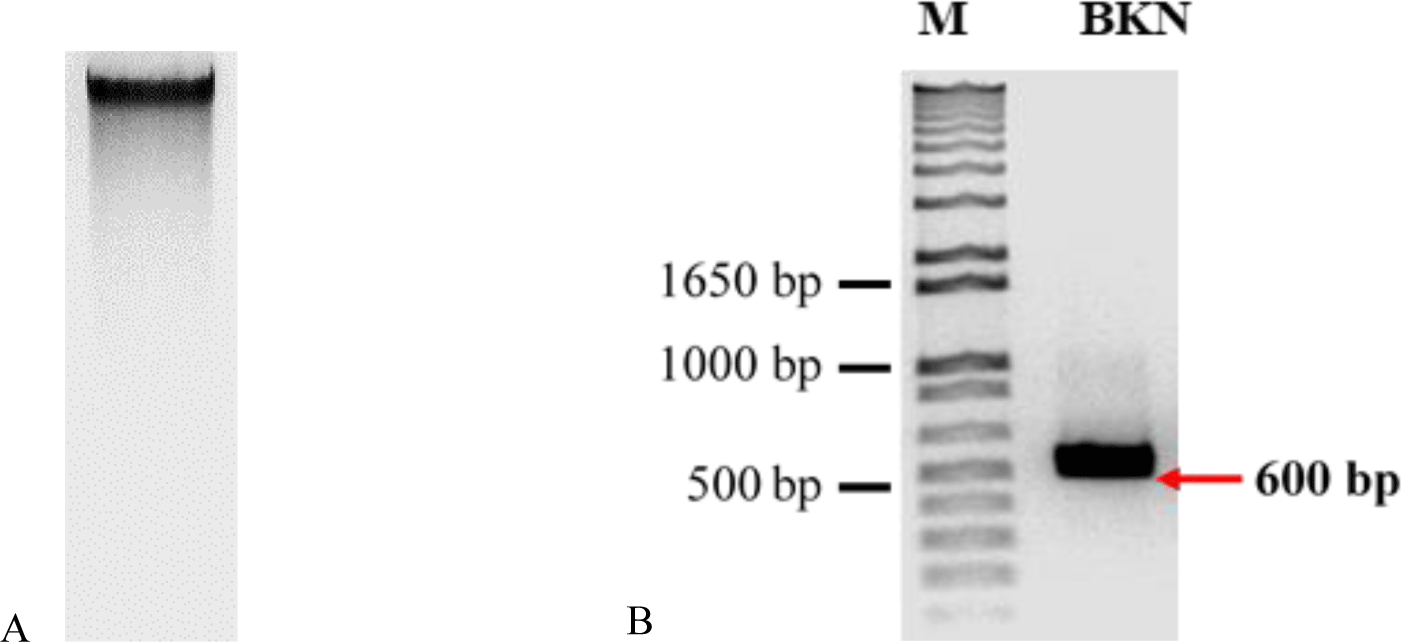
The electrophoresis image (Figure 6A) shows that the integrity of DNA extracted from fresh leaf was intact. Therefore, we used this DNA to amplify with designed rbcL primers. The rbcL primer successfully amplified the rbcL segment; the PCR product has a size of 600 bp (Figure 6B).
The data of DNA sequencing of H. formicaum was analyzed by BioEdit 7.0.5: GTAAAA-TCCAGTCCACCACGAAGGCATTCATAAACTGGCTCT ACCGTAGTTTTTAGCGGATAAACCTAATTTAGGTTT AATAGTACATCCCAACAGGGGACGACCATACTTGT TCAATTTATCTCTCTCGACTTGAATGCCGTGAGGCG GACCTTGGAAGGTTTTAACATACGCAATGGGAATTC GCAAATCTTCCAGACGCAGAGCACGCAGGGCTTTG AACCCAAATACATTACCTACAATAGAAGTAAACAT GTTAGTAACAGAACCTTCTTCAAAAAGGTCTAAGG GGTAAGCTACATAAGCAATATATTGATTTTCTTCTC CGGCAACTGGCTCAATATGGTAGCATCGCCCTTTGT AACGATCAAGACTGGTAAGCCCATCCGTCCATACA GTTGTCCATGTACCAGTAGAAGACTCGGCAGCTACC GCGGCCCCAGCTTCTTCCGGCGGAACTCCAGGTTGG GGAGTTACTCGGAATGCTGCCAAGATATCAGTATCT TTGGTTTGGTATTCAGGAGTATAATAAGTCAATTTG TACTCTTTAACACCAGCTTTGAACCCAACACTTGCT TTAGTCTCTGTTTTGGGGTGACATA.
Compared to the sequence of the chloroplast rbcL gene of H. formicarum published on Genbank [15], the result is shown in Table 1.
| Sample | Comparison of BLAST data with NCBI one | Reference | ||
|---|---|---|---|---|
| Similar species | Number code | % similarity | ||
| BKN | Hydnophytum formicarum Jack. | X81099.1 | 99 | Manen and Natali, 1995 |
The similarity between the sequence of the rbcL fragment of the tested H. formicarum leaf sample and the control one (code X81099.1) [16] on Genbank was 99%. This result confirmed that plant samples collected from Phu Quoc Forest in Kien Giang, Vietnam were Hydnophytum formicarum Jack. Therefore, we could use these plant samples for further phytochemical, pharmacognostic and pharmacological studies.
Preliminary phytochemical screening is an important step in the bioactive analysis procedure that gives a brief summary of the phytochemical characteristics of the targeted plant. The results showed that there was the presence of carotenoids, triterpenoids, flavonoids, phenolics, tannins, saponins, reducing compounds and amino acids. Alkaloid, glycoside, coumarin and fat were not detected in H. formicarum tuber (Table 2).
The average of moisture content, total ash value and acid insoluble ash value of tuber powder were 11.06 ± 0.01 (%), 9.60 ± 0.70 (%) and 0.70 ± 0.06 (%), respectively. These results met the requirements of Vietnamese Pharmacopoeia for raw medicinal materials.
The present study qualified and quantified phenolic compounds in H. formicarum tuber. The phenolic compound’s presence shows by the color changes after the extract was mixed with 2% FeCl3, 20% NaOH or 1% (CH3COO)2Pb (Figure 7). All identification reactions were positive; thus, there was the presence of phenolic compounds in the H. formicarum tuber.
4. DISCUSSION
The macroscopic characteristics of H. formicarum grown in Phu Quoc Forest are similar to those described by Vo et al. [3]. The identification of medicinal plants based on botanical characteristics is difficult, especially for species with high similarities in morphological characteristics. Therefore, DNA-based approaches have been used for the quality control of herbs and herbal products. DNA barcoding which is based upon short and standardized gene regions known as barcodes is a quick, cos-effective and species-level technique to identify herbal materials [16]. Moreover, for wild H. formicarum in Phu Quoc forest, it is usually found and/or collected without flowers, fruits and seeds. Thus, this work studied its DNA fingerprint to identify the plant. The similarity between the sequence of the rbcL fragment of the tested H. formicarum leaf sample and the control one (code X81099.1) [16] on Genbank was 99%. This result confirmed that plant samples collected from Phu Quoc Forest in Kien Giang, Vietnam were H. formicarum Jack. Therefore, DNA-based approaches based on the rbcL fragment can be used for the identification of different species of Hydnophytum distributed throughout Vietnam.
The preliminary phytochemical screening indicated the presence of carotenoids, triterpenoids, flavonoids, phenolics, tannins, saponins, reducing compounds and amino acids. These phytoconstituents may contribute to the various medicinal as well as nutritional properties of the plant. These results corresponded to the previous report of Prachayasittikul et al. (2008) in which some flavonoid and phenolic compounds in the extracts from H. formicarum tuber expressed the cytotoxic, antioxidative and antimicrobial activities [4]. Senawong et al. (2013) reported that ethanolic and phenolic-rich extracts from H. formicarum tuber possessed histone deacetylase (HDAC) inhibitory activity to inhibit proliferation of 5 human cancer cell lines mediated by induction of apoptosis [5]. The present study indicated phenolic compounds are present in the H. formicarum tuber with the total content of 58.847 ± 1.697 mg/g dried tuber powder as pyrogallol equivalent, corresponding to about 6% (w/w). Phenolics are responsible for a wide set of pharmacological properties; thus these compositions are present in most drugs used in phytotherapy [17]. This suggests that H. formicarum tuber could be a medicinal plant rich in potent biological agents to use for further studies on developing novel functional foods or new drugs.
5. CONCLUSION
The present study provided information about the botanical, genetic and phytochemical characteristics of H. formicarum growing on Phu Quoc Island, Vietnam. Its tuber contains phenolics, flavonoids, tannins, triterpenoids, saponins, amino acids and reducing compounds. These results could be useful for the authentication of H. formicarum as medicinal material used in diseases prevention and treatment.