1. INTRODUCTION
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in women of reproductive age. The prevalence of PCOS is estimated to be between 4% and 15% of women of reproductive age, depending on the diagnostic criteria used [1-3]. PCOS usually includes signs and symptoms such as hyperandrogenism, ovarian dysfunction and polycystic ovary morphology [4]. However, the pathogenesis of this syndrome is relatively complex and remains controversial. Approximately 50% of women with PCOS have accompanying metabolic syndrome [5]. Women with PCOS often have insulin resistance and may therefore be at increased risk of developing gestational diabetes mellitus (GDM) and type 2 diabetes mellitus [6].
A number of studies show an increased risk of GDM in women with PCOS [7-9], but few have studied this association in Vietnamese women. The prevalence of GDM in women with PCOS ranges from 18.2% to 54.9%, depending on age, race, GDM diagnostic criteria used and dominant PCOS phenotype [1, 3, 7, 8]. The Rotterdam criteria recognize four PCOS phenotypes: phenotype A - Frank PCOS: oligo- ovulation (OA), hyperandrogenism (HA), and polycystic ovaries (PCO), phenotype B - Non-PCO PCOS: OA, HA, and normal ovaries, phenotype C - Ovulatory PCOS: HA, PCO, and regular menstrual cycles, and phenotype D - Mild or Normo-androgenic PCOS: OA, PCO, and normal androgens [4]. The long-term disease outcomes in different PCOS phenotypes are being studied. There is evidence that women with hyperandrogenic PCOS phenotype increase risks of endocrine and metabolic abnormalities [10-12].
Current evidence suggests that Asian women have lower body mass index (BMI), fewer symptoms of hyperandrogenism and different dominant PCOS phenotype than European women [10, 13, 14], but metabolic disorders are more prevalent among Asian women than women of other ethnicities [15]. Therefore, there is a need for a separate study in Vietnamese women in order to determine the risk of GDM in women with PCOS in this specific population.
2. MATERIALS AND METHOD
This retrospective cohort study was performed at My Duc Hospital, Ho Chi Minh City, Vietnam, between January 1, 2014 and December 31, 2017.
Included women were those aged 18-38 years who conceived through assisted reproductive technology (ART) and underwent a routine 75g oral glucose tolerance test (OGTT) between 24 and 28 weeks’ gestation. Exclusion criteria were a history of pre-existing diabetes or previous GDM, hypertension, coronary artery disease, congestive heart failure, dyslipidemia, or hypothyroidism.
Information on the following pre-pregnancy and early pregnancy variables was collected for each enrolled woman: age, presence or absence of PCOS, PCOS phenotype, pre- pregnancy BMI, type of infertility, duration of infertility, ART indications, type of ART, antral follicle count (AFC), anti-mullerian hormone (AMH), first-trimester fasting plasma glucose (FPG), and whether there was a singleton or multiple pregnancy.
A diagnosis of PCOS was made using the Rotterdam 2003 criteria [4] when at least two of the following three criteria were met: polycystic ovaries, oligo-ovulation, and hyperandrogenism. Polycystic ovaries (PCO) were defined as the presence of 12 or more follicles in each ovary measuring 2–9 mm in diameter, and/or increased ovarian volume >10 ml. Oligo-ovulation (OA) was defined as a menstrual cycle length of >35 days. Hyperandrogenism (HA) was defined based on biochemical hyperandrogenism: a free testosterone index (FTI) >6 nmol/L. FTI was calculated from the measurement of sex hormone binding globulin (SHBG) and total testosterone. FTI = Total testosterone (nmol/L) x 100/ SHBG (nmol/L).
Four PCOS phenotypes were identified based on the Rotterdam 2003 criteria: A (HA+OA+PCO), B (HA+OA), C (HA+PCO), and D (OA+PCO).
GDM was diagnosed according to the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria [16] with patients required to meet at least one of the following criteria: fasting plasma glucose level 5.1 mmol/L, 1-hour plasma glucose level 10.0 mmol/L, and 2-hour plasma glucose level 8.5 mmol/L (16).
This study was approved by the Institutional Review Board of the University of Medicine and Pharmacy at Ho Chi Minh City and My Duc Hospital. Patient information was kept confidential. Informed consent was not required for this study.
Data were analyzed using Stata statistics version 13.0 (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP). Women with PCOS and those without PCOS were compared using the chi-squared test for categorical variables and the t-test for continuous variables. Multivariable logistic regression models were used to examine the association of GDM and risk factors (PCOS and first-trimester FPG) after adjusting for the following confounders: age, pre-pregnancy BMI, type of infertility, ART indications, and type of ART.
The significant difference was set at p value <0.05.
3. RESULTS
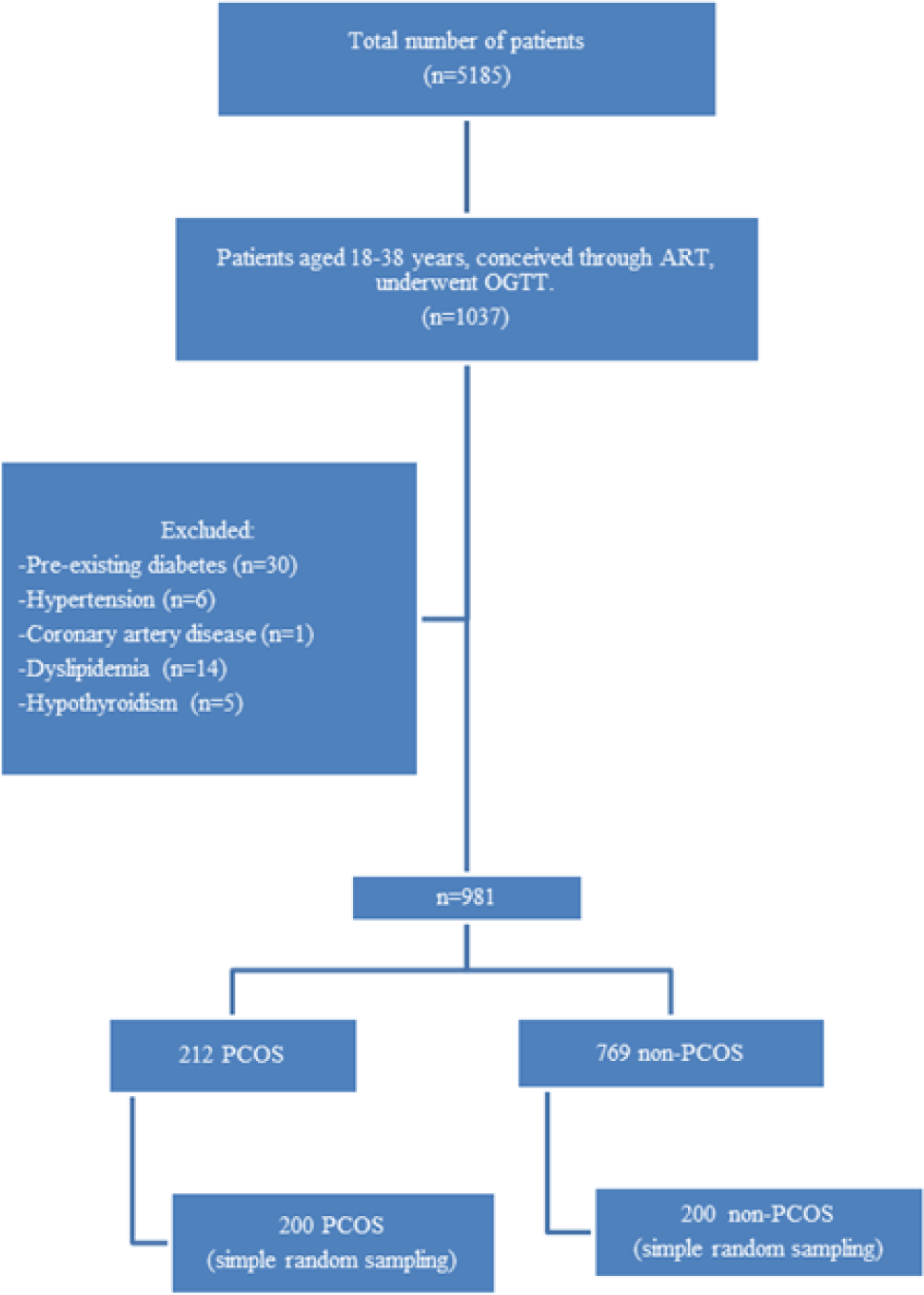
From January 2014 to December 2017, a total of 5185 patients presented to My Duc Hospital, of whom 1037 patients were aged 18-38 years, had conceived through ART and had undergone a routine 75g OGTT. After excluding 30 cases of pre-existing diabetes or previous GDM, six cases of hypertension, one case of coronary artery disease, 14 cases of dyslipidemia, and five cases of hypothyroidism, there were 981 eligible patients. This included 212 patients diagnosed with PCOS according to the Rotterdam 2003 criteria, and 769 without PCOS. From each of these two groups, 200 cases were randomly selected. Figure 1 shows the flow chart of case selection and exclusion.
General characteristics of patients according to PCOS status are presented in Table 1. Women with PCOS were significantly younger than those without PCOS. The most common ART indication for women with PCOS was ovulatory disorders, with unexplained infertility the most common for women without PCOS. Women with PCOS had higher AFC and AMH than those without PCOS. There were no significant between group differences in BMI, type of infertility, duration of infertility, type of ART, singleton or multiple pregnancy, or first-trimester FPG.
GDM was present in 37% of women in the PCOS group, compared with 26.5% in the group of women without PCOS (risk ratio [RR] 1.4, 95% confidence interval [CI] 1.04 - 1.87, p=0.02) (Table 2). Women with a diagnosis of PCOS had a higher risk of GDM than women with no such diagnosis.
| GDM | PCOS (n=200) | No PCOS (n=200) | Risk difference (95% CI) | Risk ratio (95% CI) | p-value |
|---|---|---|---|---|---|
| GDM | 74 (37%) | 53 (26.5%) | 0.105 | 1.40 | 0.02 |
| No GDM | 126 (63%) | 147 (73.5%) | (0.01-0.20) | (1.04-1.87) |
The most common PCOS phenotype in this study was phenotype D (OA+PCO; 56%), followed by phenotype A (HA+OA+PCO; 32.5%), phenotype C (HA+PCO; 7.5%) and phenotype B (HA+OA; 4%). Women with phenotype C had the highest prevalence of GDM (60%), but the differences in prevalence of GDM did not differ significantly between phenotype groups (p=0.28; Table 3).
| PCOS phenotype | n (%) | GDM | No GDM | p value |
|---|---|---|---|---|
| A | 65 (32.5) | 24 (36.92%) | 41 (63.08%) | 0.28 |
| B | 8 (4.0) | 3 (37.5%) | 5 (62.5%) | |
| C | 15 (7.5) | 9 (60%) | 6 (40%) | |
| D | 112 (56.0) | 38 (33.93%) | 74 (66.07%) |
In the crude analysis, factors that affected the risk of GDM were pre-pregnancy BMI, PCOS and first-trimester FPG. To assess whether the increased risk for GDM remained after adjusting for potential confounders, multiple logistic regression analysis was conducted (Table 4). Pre-pregnancy BMI was no longer a significant risk factor for GDM after adjusting for potential confounders.
Women with PCOS undergoing ART had a higher risk of GDM after adjusting for differences in age, pre-pregnancy BMI, type of infertility, ART indications, and type of ART (adjusted odds ratio [aOR] 2.04, 95% CI 1.06-3.92). First-trimester FPG was also an independent predictor for GDM (aOR 1.54, 95% CI 1.01-2.34).
4. DISCUSSION
In this study, the prevalence of GDM in women with PCOS was significantly higher than in those without PCOS (37% vs. 26.5%, p=0.02). These results contradict a recently published small study in Vietnam [17], which found that the prevalence of GDM did not differ between women with PCOS and those without PCOS (33.7% vs 22.7%, p>0.05), possibly because of lack of statistical power (98 women with PCOS vs. 119 without PCOS). However, the increased prevalence of GDM in women with PCOS that was observed in this study is consistent with the results of several studies [1, 3, 7, 8], all of which concluded that women with a diagnosis of PCOS had a higher risk of GDM than women with no such diagnosis.
The most common PCOS phenotype in this study was phenotype D (OA+PCO), followed by phenotype A (HA+OA+PCO), phenotype C (HA+PCO), and phenotype B (HA+OA), which is consistent with a study conducted in Indonesian women [18]. The current study did not reveal any statistically significant differences in the prevalence of GDM between the four phenotype groups, a finding similar to some previous studies [19-20]. However, there have been studies that have found the prevalence of GDM to be significantly higher in the PCOS phenotypes with hyperandrogenism compared to normo-androgenic PCOS [11, 12, 21]. This study confirms, along with a study conducted in Indonesian women, that South East Asian women are less hyperandrogenic than Western women [10, 13, 18, 22]. To our knowledge, this is the first study comparing the prevalence of GDM between four PCOS phenotype groups in Vietnamese women.
However, our sample size was relatively small for each phenotype group, therefore, there is a need for a larger study in Vietnamese women, to further investigate GDM risk in different PCOS phenotypes.
In the crude analysis, factors that affected risk of GDM were pre-pregnancy BMI, PCOS and first-trimester FPG. However, after adjustment for potential confounders, BMI no longer significantly affected GDM. Vietnamese women with PCOS tend to be lean [23-24]. A study in Chinese women, found that lean women with PCOS had a higher risk of GDM than lean controls [8]. However, research has also shown that a pre-pregnancy BMI >25 was the most important predictor for GDM [7], although in this study PCOS was also a statistically significant risk factor for GDM in women with a high pre-pregnancy BMI.
Women with PCOS had a higher risk of GDM after adjustment for potential confounders (age, pre-pregnancy BMI, type of infertility, ART indications, and type of ART), indicating that PCOS is an independent risk factor for GDM. This is consistent with previous studies, with PCOS shown to be an independent predictor of GDM (aOR=2.9, 95% CI 2.0-4.1) [8], or to be a significant risk factor for the development of GDM [1, 25]. First-trimester FPG was also an independent predictor for GDM, which is also consistent with previous studies [26-27].
There are several advantages of this study. First, this retrospective cohort study followed patients from their first diagnosis of PCOS to the subsequent development of GDM. Additionally, strict criteria were employed for the diagnosis of PCOS, and patients were classified as having GDM on the basis of objective laboratory examinations rather than by a physician’s subjective diagnosis. Furthermore, all the women in our study conceived through ART, eliminating the confounding elects of ART on the development of GDM.
Our study has some limitations. As this was a retrospective cohort study, we were unable to accurately assess clinical hyperandrogenism, relying instead on biochemical indicators. We were also unable to collect information of other risk factors: first-degree relative with diabetes, socio- economic status and smoking. Additionally, the retrospective design is associated with limitations in terms of completeness of record keeping and the results might not be representative for our study population.
5. CONCLUSION
This study revealed that among women undergoing ART, women with a diagnosis of PCOS are at increased risk of GDM compared to those without PCOS. PCOS was a significant risk factor for GDM, independent of age, pre- pregnancy BMI, type of infertility, ART indications, and type of ART. Women with PCOS should be informed of an increased risk of GDM and may require closer antenatal surveillance. There were no differences in the prevalence of GDM between the four PCOS phenotypes although larger sample-size studies may be needed to validate this finding.