1. INTRODUCTION
Staphylococcus aureus (S. aureus) is widespread colonizer of human body surface of which the anterior nostrils is the most frequent carriage site [1]. Studies have shown that Staphylococcal nasal carriage is a potential risk of community-acquired (CA) Staphylococcal infections which are a danger of public health [2-5]. In addition, the global increasing resistance of S. aureus to various antibiotics complicates treatment for its infections [6], among those methicillin-resistant S. aureus (MRSA) infections are always of most serious and difficult- to- treat ones [7-9]. In this study, we aimed to figure out the prevalence of S. aureus nasal carriage in southern Vietnamese community, associating the potential human risk factors with S. aureus nasal carriage and revealing antibiotic susceptibility of the nasal isolates which could later affect the outcome of CA-Staphylococcal infections treatment.
2. MATERIALS AND METHOD
From September to December 2013, volunteers from different places in Ho Chi Minh City, Vietnam were recruited for the study following 2 groups of age: Group 1, 18 – 35 years old and Group 2, over 59 years old, with the proportion of three (Group 1) to one (Group 2) following the age ratio of Vietnamese population (General Statistics Office of Vietnam, 2011). Persons who were having fever at the time of sampling or hospitalized in the previous month were excluded.
Sample was collected from nasal cavity by rotating a sterile swab in the nares of each participant. Amies transport medium with charcoal (TITAN MEDIA, India) was used to carry samples to the laboratory for culture and identification. At the same time, the information on age, gender, career, height, weight, history of antibiotic usage in the past 2 months, daily nasal wash with water, use of nasal medication sprays, acne problems, smoking, nasal problems (such as asthma or sinusitis) and history of S. aureus infection of every participant was also collected.
Samples were cultured on mannitol salt agar (MSA; HiMedia, India). All colonies surrounded by yellow zones on MSA after 24 hours of incubation at 37°C were selected for identification using Staphylase test kit (Oxoid, UK).
Sensitivity test was performed followed the Kirby-Bauer disc diffusion method. Inoculum used for the test was prepared from 1-3 colonies picked from culture plate and suspended in 5mL sterilized Mueller Hinton Broth (MHB; HiMedia, India) and adjusted for proper cell density using optical density at 600 nm. Antibiotic discs (Nam Khoa Biotek, Vietnam) used in this test included: ampicillin (10μg), cephalexin (30μg), meropenem (10μg), kanamycin (30μg), erythromycin (15 μg), clindamycin (2μg), tetracycline (10μg), vancomycin (30μg), ciprofloxacin (5μg) and linezolid (30μg). Diameter of inhibition zones was recorded in millimeter after 24 hours of incubation at 37oC. The antibiotic susceptibility was interpreted via CLSI guidelines [10].
Statistical Package for the Social Sciences (SSPS) for Windows (Version 16.0) software was used to statistically analyze the association between risk factors and S. aureus nasal carriage. The results were presented with 95% confidence interval (CI) and corresponding p value. The level of significance was set at 0.05 using the two-tailed method.
3. RESULTS AND DISCUSSION
In total, there were 205 volunteers joined this study. These comprised 96 males (46.8%) and 109 females (53.2%) with ages ranging from 18 to 94 years of which 154 were in Group 1 (18-35 years old) and 51 were in Group 2 (over 59 years old).
Percentage of nasal carriers in Group 1 is 10.4% (16/154) while Group 2 is 13.7% (7/51). Overall, nasal carriage of S. aureus in this study population was 11.2% (23/205) which is lower than a study in urban and rural northern Vietnam (15.8%, 161/1016 for nasal carriage and 29.7% for nasopharyngeal carriage, 302/1016) which was carried also with young people (35% of the cohort < 20 years old) who generally have markedly higher rate of nasal carriage [11]. This percentage is also slightly lower than a recent study carried out on 838 patients at an ICU in southern Vietnam (13.1%) [12]. In addition, in our study carriage prevalence was similar between males and females (11.5% and 11.0%) which were in agreement with previous study [11]. It seems that even though this study has relatively small sample size, the result still reflected quite well the status of big population. The rate of S. aureus nasal carriage in relationship with some risk factors is shown in Appendix A.
There was not any significant effect of the risk factors to the rate of S. aureus carriage in both groups based on data analysis (data not showed) even though differences in the percentages of S. aureus carriage were observed in each group of age (Appendix A).
Potential risk factors for nasal S. aureus carriage have been studied but none of them were significant. It is probably due to the fact that our sample size was too small for each factor to provide a significant value. For example, with the factor of the history of using antibiotic during last 2 months, it was observed that percentage of carriers in both groups was lower for antibiotic using people (7.7 % compared to 12.1 %) but still not statistically significant (analysis not shown). It has been shown that smokers were less likely to become S. aureus carrier than non-smokers [13] but in our study a similar pattern could only be found in group 1 but not in group 2 due to limited sample. Other factors such as cleaning nose with water or nasal spray and health conditions such as obesity or sinusitis and asthma problems which have been shown to have relationship with S. aureus colonization did not achieve significant level in our study [14].
In this study, resistant rate of S. aureus isolates were found to be 95.7% for ampicillin, 34.8% for cephalexin, erythromycin, kanamycin, and clindamycin, 47.8% for meropenem, 17.4% for tetracycline, 21.7% for ciprofloxacin and 4.3% for linezolid. Especially, vancomycin continued to be the treatment of choice for treating most MRSA infections with none of isolates (0%) resistant to this agent. The data was summarized in Figure 1. The prevalence of multi-drug resistance (resistant to at least 3 tested antibiotics) of S. aureus isolates was shown in Figure 2. There were thirteen isolates with resistance to at least 3 antibiotics of which two isolates were identified to be resistant to 8 antibiotics. Only one isolate was susceptible to all antimicrobial agents.
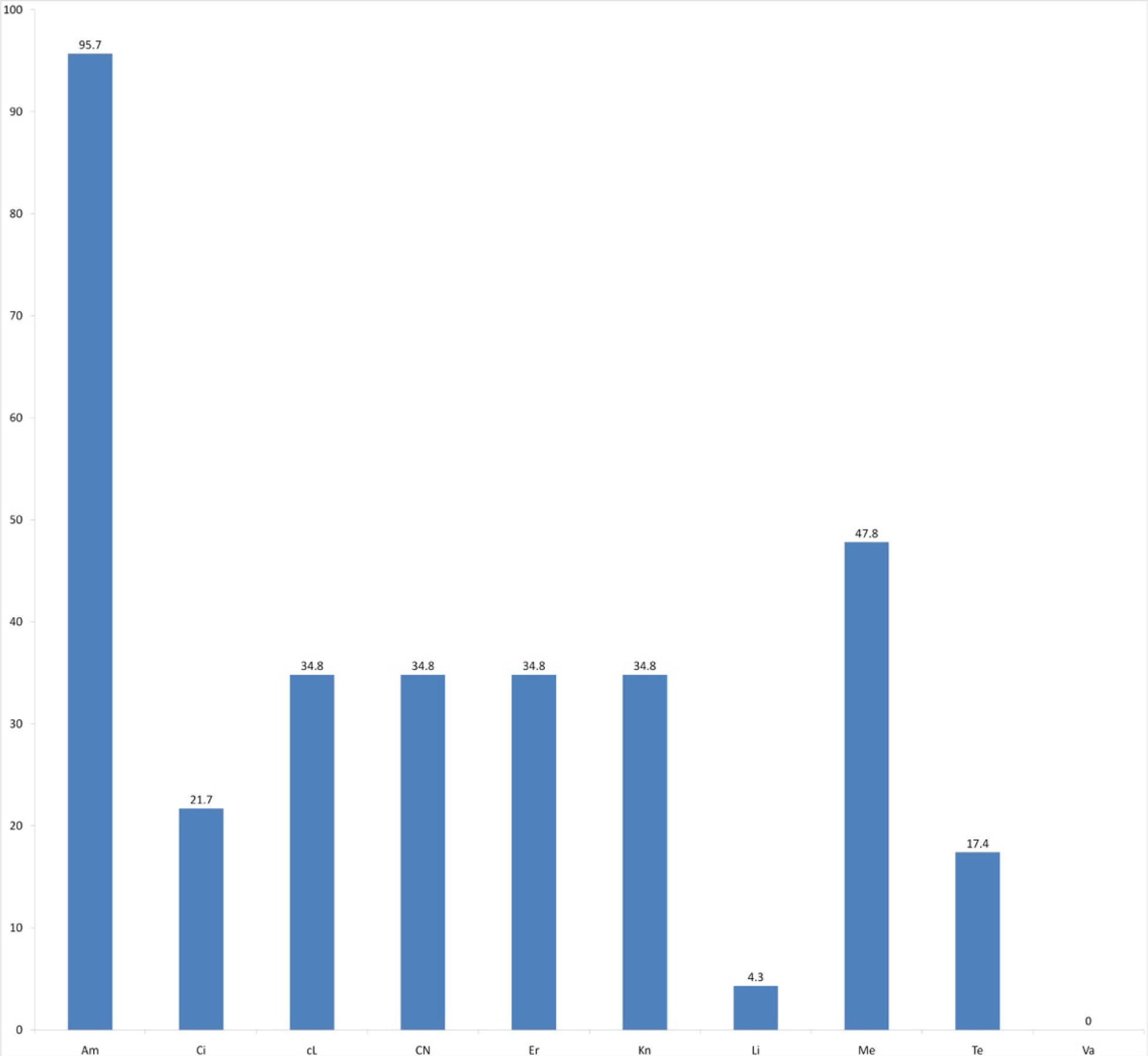

With the high resistance rate, ampicillin, meropenem and even cephalexin, clindamycin, erythromycin and kanamycin were not suggested for S. aureus infections treatment. It is different to recent study showing that first generation cephalosporins (cefazolin, cephalothin and cephalexin), clindamycin and erythromycin have important therapeutic roles in less serious S. aureus infections such as skin and soft tissue infections or in patients with penicillin hypersensitivity (urticaria, angioedema, bronchospasm or anaphylaxis) [15]. Besides, in our study, ciprofloxacin and tetracycline are two antibiotics to which S. aureus has low resistant rate (21.7% and 17.4%, respectively). Our results also suggested that linezolid and vancomycin remain important in the treatment of S. aureus infections because of high susceptible rate– 95.7% and 100%, respectively. It is in agreement with previous results showing that vancomycin has been considered the treatment of choice for infection due to S. aureus [16].
Multidrug resistance was frequently observed in this study with thirteen (54.2%) of all the isolates resistant to at least three antibiotics. Only one isolate (4.2%) was fully susceptible to all the tested antimicrobial drugs. The multidrug-resistant S. aureus rate is comparable to what was reported in Nigeria (52.5%) [17] and lower than in other countries – 75% in Bangladesh [18] and over 94% in India [19]. However, compared to developed country, multidrug resistant S. aureus rate in this study is markedly higher – 32% in The USA and 24.6% in Europe [20-21].
4. CONCLUSION
In our study, some risk factors influenced S. aureus nasal carriage rate when analyzing them in different age groups but not in the whole population. Results also indicated that rate of S. aureus nasal colonization can be reduced via improving personal hygiene, such as performing hand washing frequently and effectively.
The study showed low rate of S. aureus nasal carriage among healthy Vietnamese but a high prevalence of multi-drug resistance in nasal S. aureus isolates. Resistance to beta-lactams and commonly prescribed antibiotics was dominant. Data suggested that it is important to control multi-drug resistance not only in healthcare systems but also in community to prevent the infections potentially caused by these multi-drug resistant isolates.








