1. INTRODUCTION
According to the World Health Organization, around 7.7 million cases of glaucoma led to moderate-to-severe vision impairment and blindness in 2020 [1]. This disease is categorized into two subtypes, namely open-angle glaucoma and angle-closure glaucoma, with the latest global prevalence of 3.05% and 0.5%, respectively [2]. The number of glaucoma cases in the 40–80-year-old age group was predicted to reach 111.8 million by 2040, among which approximately 79.76 million would suffer from primary open-angle glaucoma (POAG) [2]. Presenting the highest number of POAG and angle-closure glaucoma cases (23.54 million and 15.47 million respectively), Asia, followed by Africa, plays a significant role in the global management of glaucoma, and poses the urging necessity for the development healthcare system in glaucoma screening and treatment [2].
The current consensus guideline offers three approaches for glaucoma therapy including medication therapy, laser procedures and surgery, with the treatment objectives of preventing or deferring disease progression and complications on optic nerve system, maintaining vision and quality of life for patients [3–6]. The most common treatment option is medication therapy, in which fixed-dosed combination (FDC) eye-drop products gain fondness for the benefits of simplifying treatment regimen and improving patient adherence. According to the 4th Guideline by Asia Pacific Glaucoma Society in 2024, FDC formulation enhances the ease of use for patients since it is only required one drop each use, instead of multiple drops from different packages, which lowers the risk of improper regimen use [5]. In their systematic review and meta-analysis publication, Wei et al showed that FDC therapy significantly improved the medication compliance of patients by 1.29 times (95% CI: 1.23–1.35, p<0.001) comparing to free-equivalent combination therapy (FEC) [7].
In Vietnam, according to Circular No. 20/2022/TT-BYT, only two FDC regimens for glaucoma, namely prostaglandin analogues (PGA)+Timolol and carbonic anhydrase inhibitors (CAI)+Timolol, are reimbursed by health insurance. Based on clinical practice, expert consultations, and treatment guidelines, the CAI+Timolol group is frequently used for patients who require additional intraocular pressure (IOP) reduction after monotherapy with β-blockers or who do not tolerate PGA therapy. Although PGA+Timolol combinations are also reimbursed, they differ significantly in the mechanism of action, clinical indication, and patient profile, and are often reserved for cases requiring more aggressive IOP-lowering effects. In addition, FECs were excluded due to lower adherence and inferior cost-effectiveness compared to FDCs [5,7]. As such, Brinzolamide+Timolol (BTFC) was selected as the most clinically relevant and appropriate comparator for DTFC in this context. The two popular FDCs of CAI and Timolol are Dorzolamide+Timolol (DTFC) and BTFC. Though evidence on the comparative clinical efficacy of DTFC and BTFC are available, there are lack of publication in Vietnam on the pharmacoeconomic comparison of the two in treatment for glaucoma and ocular hypertension (OH), which raises the question of their offering in economic benefits for patients [8–10]. Thus, this study aimed to analyse the cost-effectiveness of DTFC versusBTFC in the treatments of OH/POAG in Vietnam. The results can provide insights on the economic efficiency of DTFC, which aid the health policy development and the glaucoma-therapy indication for optimal treatment effectiveness.
2. MATERIALS AND METHODS
In this pharmacoeconomic assessment of Dorzolamide+ Timolol Fixed-Dose Combination (DTFC) versus Brinzol-amide+Timolol Fixed-Dose Combination (BTFC), the target population was defined as adult patients diagnosed with OH or POAG, according to the ICD-10 diagnosis code H40.11. The population included patients with mild-to-moderate OH, encompassing both treatment-naïve and previously treated individuals. Disease severity was classified based on the POAG severity stages in the Markov model, reflecting the progression from OH to early-stage POAG. Patients were included in the model if they had OH or POAG, with or without optic atrophy or related complications, such as visual field (VF) loss. Importantly, patients without other concurrent ocular pathologies were considered for inclusion, and it was assumed that those with OH would enter the Markov model through the ‘early POAG’ stage once treatment criteria were met.
This pharmacoeconomic study applied modelling techniques, using real-world data from Ho Chi Minh Eye Hospital for the cost-effectiveness analysis of DTFC versus BTFC in the treatment of OH/POAG. Model parameters such as response rates, treatment switching, and resource use frequencies were determined through structured consultations with ophthalmologists at Ho Chi Minh Eye Hospital, and triangulated with evidence from published clinical trials and systematic reviews. This study was conducted in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) 2022 guidelines [11]. A completed checklist is provided as Supplementary Table 1.
BTFC was chosen as research comparator for the usual clinical practice and choice of treatment in Vietnamese settings, as priorly presented in the Introduction section.
Perspective of third-party payer in healthcare (Vietnam Health Insurance Fund in particular) was adopted in this analysis, accounting for direct medical costs for medication and health services incurred from laser procedures, diagnostic examinations (including VF test, tonometry, optic disc photography, retinal-optic disc tomography), hospital stays, and physical examinations. Societal costs, such as productivity loss or informal care, were not included. All cost data were collected and reported in Vietnamese Dong (VND) in 2024, reflecting the local healthcare payer’s perspective.
Incremental cost-effectiveness ratio (ICER) per quality-adjusted life year (QALY) was calculated for cost-effectiveness conclusions, which was derived from treatment effectiveness by QALY and costing estimation in 2024. This indicator is widely used for its universal implication but features a limitation in disease-specific willingness to pay threshold, which can lead to misinterpretation if not carefully examined.
A decision-tree model and a Markov model were combined to simulate treatment pathways and long-term disease progression, developed by consensus treatment guideline and validated by expert consultation. The decision-tree model captured short-term therapeutic decisions (e.g., treatment response and switching) during the initial year, which are often nonlinear and require discrete branching logic. The Markov model then simulated long-term disease progression across health states with annual cycles, which was deemed appropriate for chronic conditions like POAG. This mixed modelling approach is widely recommended in pharmacoeconomic modelling of chronic diseases and aligns with prior studies [12,13].
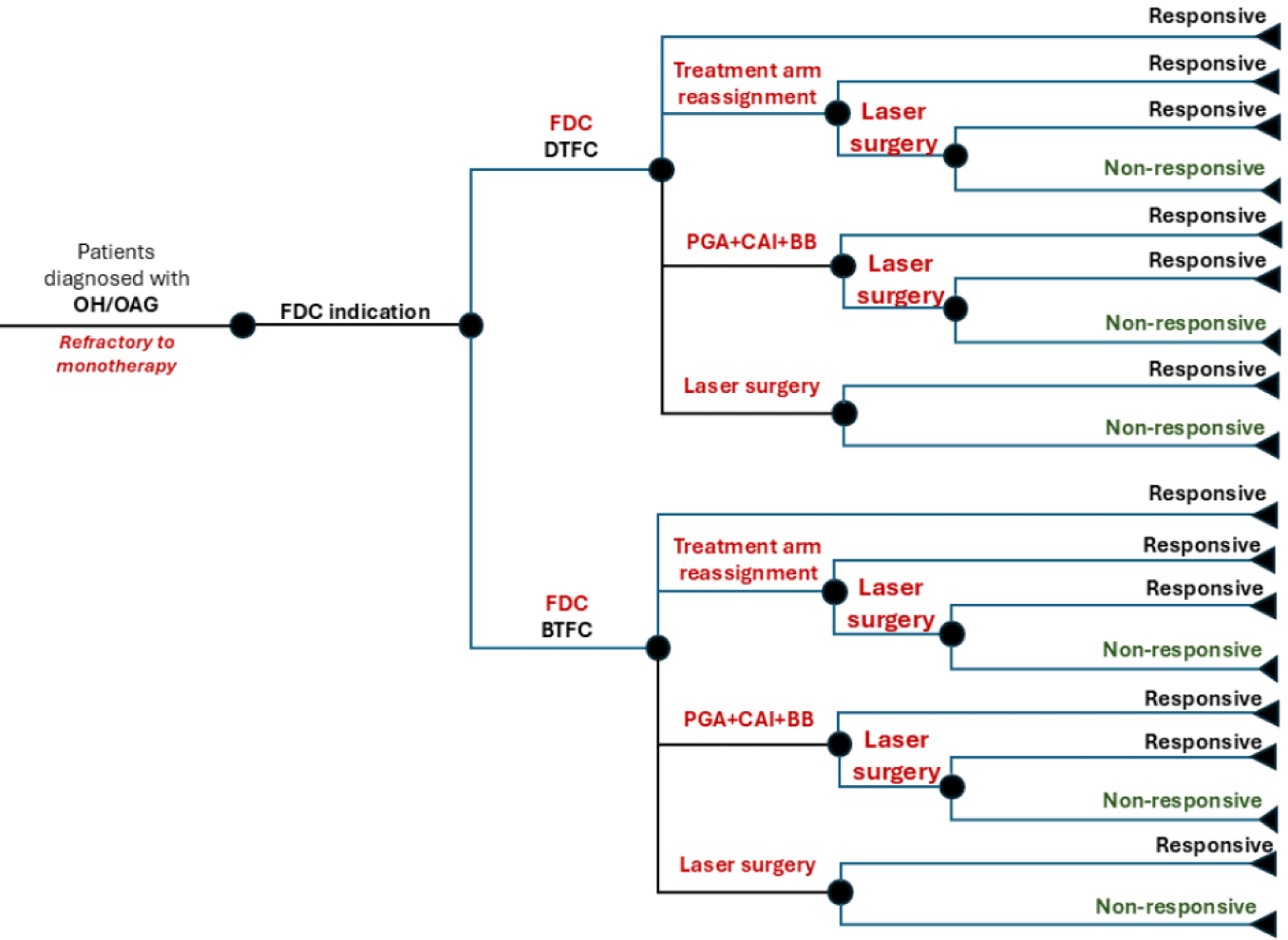
In the decision-tree model, a hypothetical cohort of 10,000 patients with OH or POAG, refractory to monotherapy, were assigned to either the DTFC or BTFC treatment arms. Patients were evaluated under two scenarios: Scenario 1 assumed treatment success with FDC, maintaining the therapy throughout the analysis period. In Scenario 2, patients unresponsive to FDC were reassigned to alternative strategies, including switching to the counterpart treatment arm, adding prostaglandin analogues, or proceeding directly to laser surgery if no response was observed (Fig. 1).
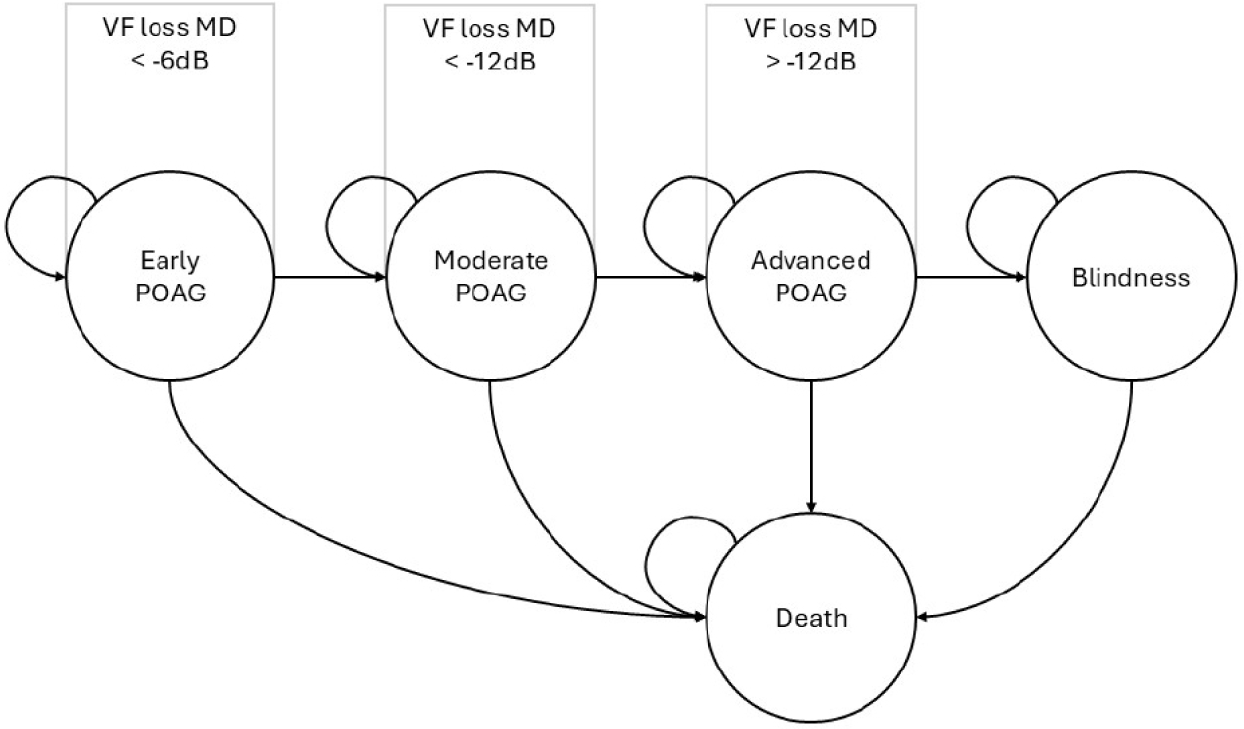
The model was designed to simulate long-term disease progression following the acute treatment. Hither, patients were put under the assumptions of continuing previously responsive treatment until the end of observation, and of absolute treatment adherence. The health states of the model were early POAG (MD<–6 dB), moderate POAG (MD<–12 dB), advance POAG (MD>–12 dB), blindness and death (Fig. 2).
The timeframe of decision tree model was twelve months with 3-month cycle, while Markov model applied lifetime timeframe with cycle duration of one year.
The value of discount rate for both cost and effectiveness parameters was 5% per year. The discount rate followed the consensus recommendation in health economics and was suitable for economic context in Vietnam [14].
In the decision-tree model, the transition probabilities for each treatment arm were determined based on IOP reduction achieved by the respective treatments. These values were derived from a meta-analysis of randomized controlled trials (RCTs) and reflect treatment-specific efficacy. To simulate treatment pathways accurately, the proportions of patients requiring treatment reassignment or escalation after becoming refractory to first-line therapy were informed by clinical expert consultation at Ho Chi Minh Eye Hospital, ensuring the model closely reflects real-world clinical practices.
In the Markov model, transition probabilities between health states were determined based on disease progression data and adjusted according to treatment effectiveness reflected by IOP reduction. The IOP levels achieved in the decision-tree model were used to estimate changes in VF, which defined each patient’s health state (e.g., early, moderate, or advanced POAG) at the start of the Markov phase. These health states then guided the transition frequencies over time. This approach allowed the model to dynamically simulate disease progression as influenced by treatment response. The transitional probability to the “death” health state was extracted from Vietnamese age- and sex-standardized mortality statistics by World Health Organization [15].
Information on transitional probability and proportion for both models are provided in Table 1.
| Parameter | Value | Distribution | Source |
|---|---|---|---|
| Years of age (mean [SD]) | 57 (17) | Lognormal | [12,13] |
| Sex ratio (Female/Male) | 49/51 | Beta | [14] |
| Baseline MD (dB, mean [SD]) | –6.2 (7.6) | Lognormal | [13] |
| Monthly MD natural reduction (dB), mean (SD) | 0.05 (0.07) | Lognormal | [15] |
| IOP reduction (% [SD]) | |||
| Latanoprost | 29.5 (13.4) | Normal | [16] |
| Timolol+Dorzolamide (concomitant) | 18 (12) | Normal | [16,17] |
| 1 active ingredient+1 active ingredient or laser surgery (concomitant) | 18 (12) | Normal | [16,17] |
| 2 active ingredients+1 active ingredient or laser surgery (concomitant) | 10 (5) | Normal | [16,17] |
| 3 active ingredients and laser surgery (concomitant) | 8 (4) | Normal | [16,17] |
| Laser surgery | 30 (12) | Normal | [18–22] |
| Comparative efficacy ratio of DTFC and BTFC (mean [SD]) | 1.03 (0.153) | Normal | [23] |
| Proportion of treatment transition after refractory to first-line therapy (% [SD]) | |||
| Transition to FDC | 67.14% (14.85%) | Clinician consultation, real-world data | |
| Third concomitant drug prescription | 17.86% (9.95%) | ||
| Laser surgery | 15.00%(10.35%) |
The vital clinical indicators based on the conclusion from literature reviews and clinical expert opinions were “the mean reduction of IOP”, “visual field damage” and “quality-adjusted life years”. These indicators were included to the models as effectiveness parameters.
Data on IOP reduction from each treatment phase were derived from meta-analysis of RCT of respective treatment [16].
The severity of VF damage was based on the VF mean deviation (MD), following the approach of Canadian Agency for Drugs and Technologies in Health in effectiveness assessment of glaucoma treatments in health economic assessment [17]. Accordingly, the change of MD was calculated as follows, using the natural disease progression (NP) of untreated glaucoma patient and the standardized reduction (SR) derived from VF damage and IOP reduction [13].
In the above formula, the annual IOP reduction in each treatment arm was calculated as the sum of the IOP reduction in one year of treatment wherein multiple interventions were implemented as simulated by the decision-tree model.
Data on the treatment effectiveness based on QALYs were derived from the systematic reviews results on utility values in respective health state representing the severity of disease. Data of utility value in each health state and their respective probability distribution are presented in Table 2.
| Health state | Utility value | Parameter distribution | Source |
|---|---|---|---|
| Early POAG | 0.847 | Beta (251, 45) | [24,25] |
| Moderate POAG | 0.781 | Beta (231, 65) | |
| Advance POAG | 0.704 | Beta (208, 88) | |
| Blindness | 0.594 | Beta (176, 120) | |
| Death | 0 |
Costs of medication were extracted from the latest “Summarized drug bidding reports of distribution facilities” of the Department of Pharmaceutical Management-Vietnam Ministry of Health by the time of research completion (Table 3).
The clinical consultation was implemented in 2024 to simulate the most updated the clinical practice of OH/POAG treatment in Vietnamese medical settings. Costs incurred for health services were calculated in Vietnam Dong before converting to USD using the exchange rate in 2024. The calculation was derived from clinician consultation and official costs of health services promulgated in Circular no. 22/2022/TT-BYT by the Vietnam Ministry of Health [18] (Table 4).
The total costs and QALYs were calculated for DTFC and BTFC over two models with respective timeframe as presented. From a payer perspective, total costs included direct medical costs (covered by third-party payers). The primary outcome was ICER defined as the difference in costs divided by the difference in QALYs of the two treatment arms. Willingness-to-pay threshold was set at 3-time of GDP per capita in Vietnam, published by the General Statistics Office of Vietnam [19].
Deterministic sensitivity analysis (DSA) served to assess the uncertainty of the models and explore the parameters to which the models were most sensitive. Each parameter, one at a time, was adjusted between corresponding 95% confidence intervals, or 20% deviation, or standard upper and lower values (such as 0% and 6% for discounting parameter of cost and effectiveness). For the probabilistic sensitivity analysis (PSA), Monte Carlo simulation method was applied with 10,000 iterations. In each of which, the model parameters were assigned value randomly drew from corresponding probability distributions to explore the robustness of the results to variations of multiple parameters at once.
The development of research objectives and design went through a thorough process of consultation from relevant stakeholders to answer the most critical question for the best choice of OH/POAG treatment that addressed the interest of both clinical practitioner and health policy makers. Furthermore, the estimation of cost and effectiveness parameters were performed by using real-world data from Ho Chi Minh Eye Hospital and offered the most relatable and applicable scenario for the implication and suggestion in Vietnamese medical setting. The results were then simplified and translated in the mutual, and less field-specific, context that can extend the coverage in the use of findings to the most relevant population possible.
3. RESULTS
The results from base case analysis of the hypothetical population showed that the total cost of treatment by DTFC was 429,066,001,437 VND, and by BTFC was 438,649,383,016 VND. The cost difference of the two arms was –9,583,381,579 VND. Regarding treatment effectiveness, DTFC arm yielded 94,955.41 QALYs at the end of analysis timeframe, higher than BTFC with 94,943.59 QALYs, offering an additional benefit of 11.82 QALYs.
Consequently, regarding the comparative analysis for each patient, cost of treatment by DTFC was 42,906,600 VND, lower than the cost of treatment by BTFC (43,864,938 VND), differed by –958,338 VND (for one year in the decision-tree model and 40 years in the Markov model). DTFC offered 9.5 QALYs for each patient, higher than BTFC (9.49 QALYs). However, the difference in quality-of-life benefits from DTFC and BTFC was insignificant with only 0.001182 QALY. While the QALY difference per patient was small (0.001182), the cost savings observed and the potential for improved adherence justify consideration in treatment policy. This indicated the lower treatment cost of DTFC compared to BTFC, while their comparative effectiveness is almost equal.
Table 5 shows the results of base-case analysis on the hypothesis population and for each patient.
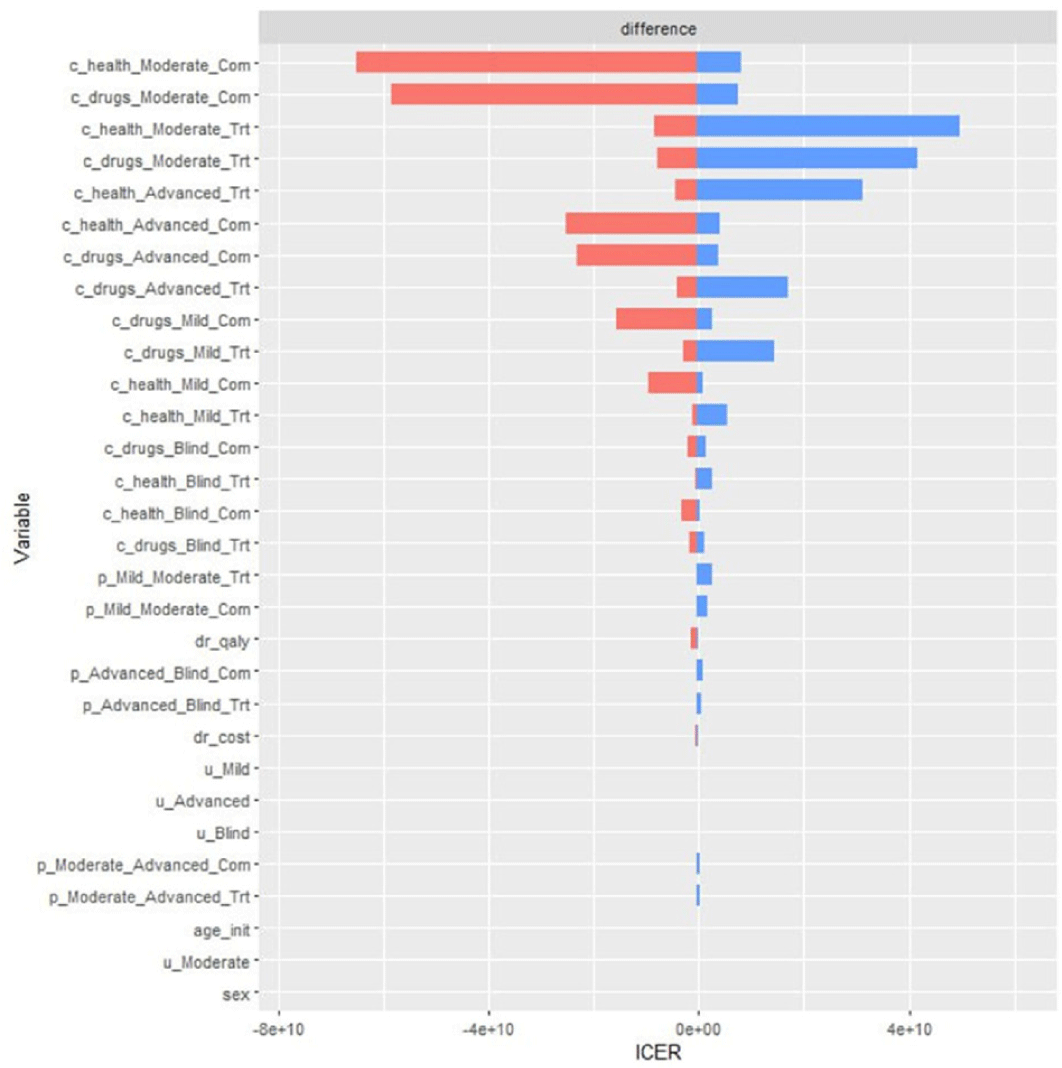
The results of DSA are presented as a tornado diagram in Fig. 3, designed to depict the change intensity of ICER as each parameter deviates. Accordingly, the models were most influenced by variation of the costs incurred for health services. The second- and third-most influencing parameters were costs for DTFC treatment, and costs for BTFC treatment in each health state in Markov model (Fig. 4).
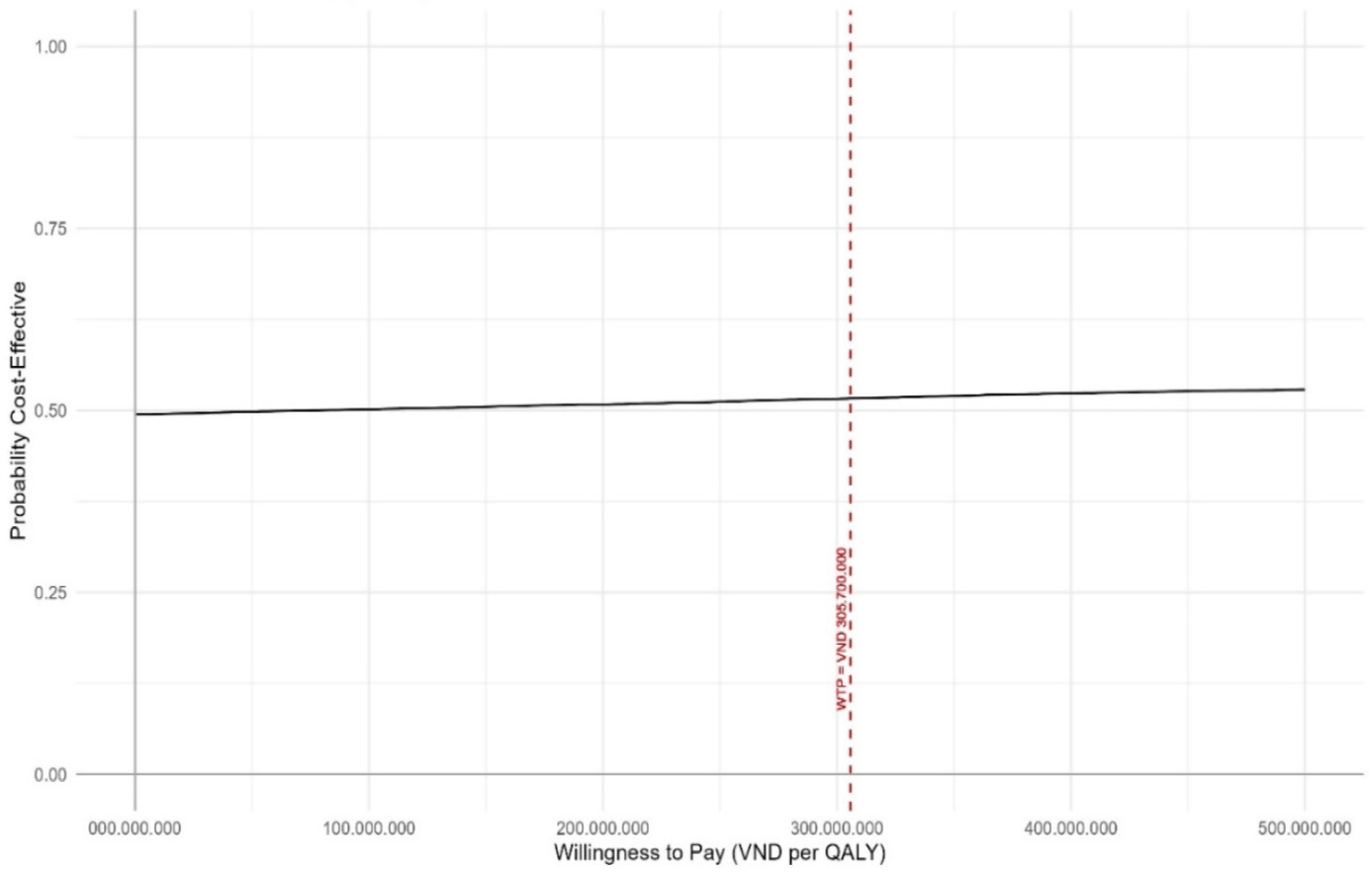
Results of PSA using Monte-Carlo 10,000-iteration simulation are presented in Figs. 4 and 5. At the willingness-to-pay of 3-time Vietnam GDP per capita in 2023 (approximately 305,7 million VND per QALY gained), DTFC demonstrated a 51.53% probability of being cost-effective compared to BTFC at the standard willingness-to-pay threshold (Fig. 5).
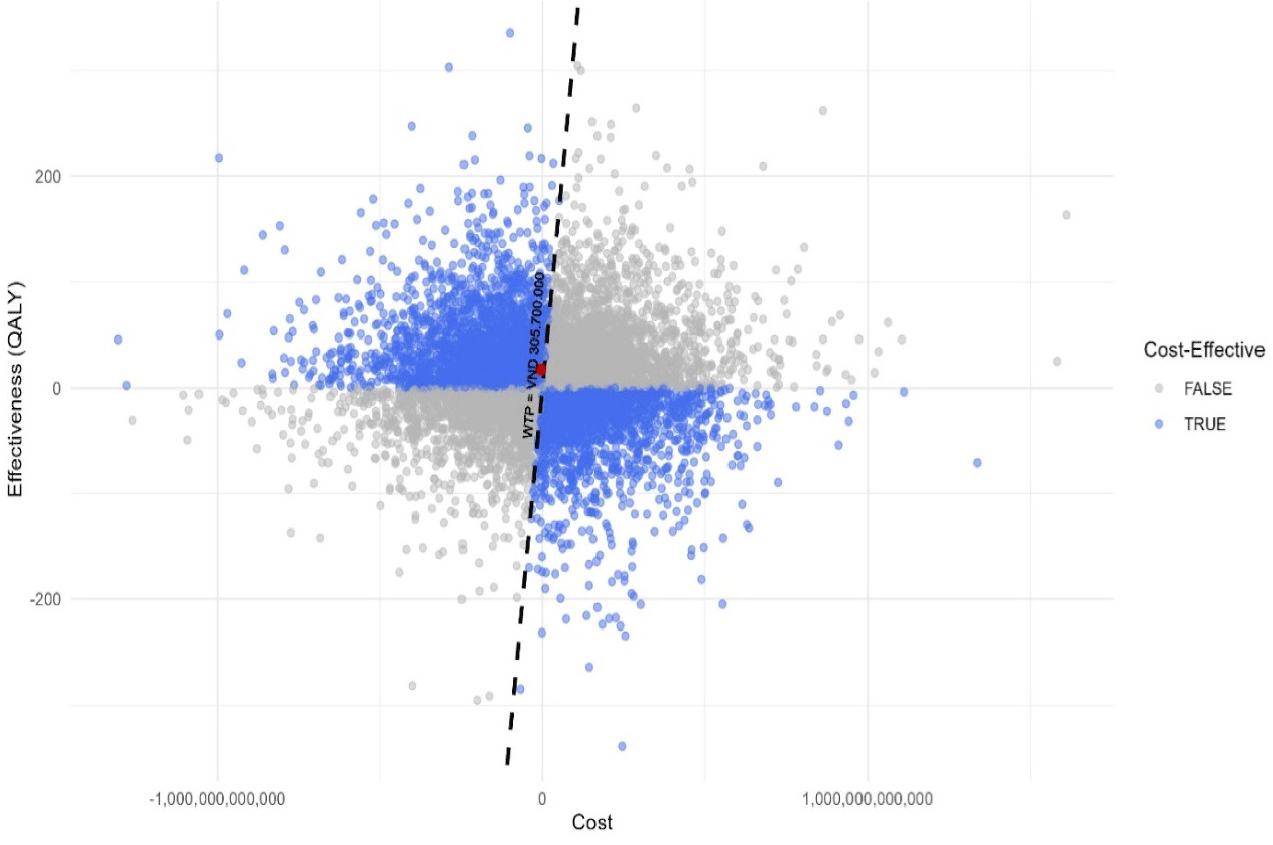
4. DISCUSSION
Results from base case analysis showed that DTFC was more cost savings than BTFC, while guaranteed treatment effectiveness since the difference was insignificant. Costs incurred for health services and medication costs were the parameters that had the most influence on the model outcome. PSA results showed that at the willingness-to-pay threshold of 3-time GDP per capital in Vietnam, DTFC had a probability of 51.53% of being cost-effective compared to BTFC. Our findings indicated the cost-saving potential of glaucoma treatment by DTFC, but the treatment selection should be carefully considered based on other associated factors and cost-effectiveness probability under the influence of abovementioned factors in Markov model.
The models applied in our pharmacoeconomic assessment were developed using literature reviews on disease progression, pharmacoeconomic models on the treatment for OH/POAG and clinician consultation [12,13]. The objectives were to propose the model with proper structure that can reflect the clinical practices in two phases: (1) the initial treatment phase depicted through the decision-tree model, and (2) the maintenance treatment phase depicted through the Markov model. This approach not only offers the compatibility with practical treatment procedure, but also simulates the NP, enabling the monitoring of unrecovered disease progression through different health states as follows: “early POAG”, “moderate POAG”, “advance POAG”, “blindness” and “death”. The analysis timeframe, including the first year in decision-tree model and the lifetime timeframe in the Markov model, offers strength in analysis, since it reflects the chronic characteristics of the disease, and yields more accurate prediction in costs for long-term care. Moreover, the real-world data for pharmacoeconomic analysis retrieved from Ho Chi Minh Eye Hospital were used to optimize the input data that can address the practical situation in Vietnam.
Comparing to our assessment, the cost-effectiveness evidence of DTFC versus BTFC from Rouland et al in 2003 and Jothi et al in 2010 were analysed in shorter timeframes (3 months and 8 months, respectively) which raised the question of their ability to reflect the long-term treatment process of the disease [20,21]. Hence, it further emphasizes the strength of our research, being one of the first studies to evaluate the cost-effectiveness of two interventions with a long-term timeframe, using input data sources that reflect clinical practice and appropriate to the treatment context in Vietnam [22].
However, there are some potential risks of bias from the data validation, hypothesis probability distribution and long analysis timeframe. This study has several limitations. First, due to the lack of large-scale clinical trials in Vietnam, treatment efficacy data were primarily sourced from international literature [23–25]. While model inputs were adjusted through expert consultation and real-world cost data from Vietnamese hospitals, generalizability may still be limited. Second, the model assumed perfect adherence, which may overestimate real-world effectiveness and cost-efficiency. Third, comorbidities were not included as covariates due to unavailable data, which may affect transition probabilities. Besides, the exclusion of indirect costs, such as management, monitoring, and patient follow-up, limited the comprehensiveness of our economic evaluation. Lastly, although the QALY difference per patient was minimal, it could lead to meaningful implications at the population level, supporting the value of cost-saving strategies like DTFC.









